Do the photochromic lenses fascinate you too? Well, for me they have always been a mysterious topic. The way the glasses acquire the tint when exposed to the sunlight, has always made me wonder, as to why the transition takes place. As a kid, owning them and showing them off to my friends had always been a matter of pride for me. Like me, there must be many who want to understand how the photochromic glasses work. So let us check out the truth behind the photochromic conundrum.
 What are photochromic lenses?
What are photochromic lenses?
The word “photochromic” is derived from two Greek words “Photos” which means Light and “Chroma” which means Colour. Thus, photochromic means the something that changes its colour in response to the Light.
History
These were originally developed in the 1960s by Donald Stookey who worked as a chemist at Corning Glass Works. Soon after Stookey patented the material, Roger Araujo, another Corning chemist, developed the first photochromic lenses.
Composition
Photochromic lenses are made up of three types of material that are glass, polycarbonate or plastic. The photochromic lenses made up of glass have microcrystalline Silver halides (AgX-most commonly chlorine) embedded in the glass.
The plastic versions were based on based on thin films of organic compounds like oxazines i.e pyridobenzoxazines, naphthopyrans or indenonaphthopyrans in them.
The reason behind the tint. 
These lenses darken or lighten dependent on their exposure to ultraviolet (UV) radiation and return to the clear state gradually when the UV exposure is removed.
These lenses darken only when exposed to the ultraviolet component of the sunlight and not in response to the artificial light whatever be the intensity of artificial light. Like tiny transformers, they can switch between either form depending on the presence or absence of UV light
The mechanism
The transition depends on the principle of equilibrium.
Chemical equilibrium – It is the state in which both reactants and products are present in concentrations that have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction.
The exposure to the UV rays disturbs the equilibrium and hence the tint appears.
 Darkening process – In most of the photochromic lenses, the molecules of silver chloride or another silver halide are embedded. The silver halides are not affected by the visible spectrum, light without the ultraviolet component and artificial lighting.
Darkening process – In most of the photochromic lenses, the molecules of silver chloride or another silver halide are embedded. The silver halides are not affected by the visible spectrum, light without the ultraviolet component and artificial lighting.
However, the molecules are very sensitive to the UV component having a wavelength of 315 nm – 400 nm. On exposure to the UV light the Silver Halide (Most commonly chlorine) molecules is oxidized to give Silver and halide molecules.
Most commonly Silver I chloride is used. On exposure the silver gains an electron from chloride to become silver metal, and gets the ability to absorb visible light and appear darker. The silver metal is mainly responsible for the dark tint or shade in the lenses.
![]()
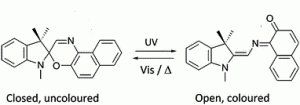 In the case of the plastic lenses, the organic photochromic molecules undergo a chemical process. The carbon molecules in the lenses such as pyridobenzoxazines, naphthopyrans, and indenonaphthopyrans—react to UVA light by rearranging their chemical bonds into a new species which causes them to change shape and absorb a significant percentage of the visible light and hence darken, when exposed to ultraviolet rays. These processes are reversible.
In the case of the plastic lenses, the organic photochromic molecules undergo a chemical process. The carbon molecules in the lenses such as pyridobenzoxazines, naphthopyrans, and indenonaphthopyrans—react to UVA light by rearranging their chemical bonds into a new species which causes them to change shape and absorb a significant percentage of the visible light and hence darken, when exposed to ultraviolet rays. These processes are reversible.
The organic films of plastic photochromic lenses are more susceptible to degradation than the silver halides used in glass, even when the plastic lenses are lighter and thinner than their glass counterparts.
 Clearing process – The darkening process is almost instantaneous. However, the lenses become clear in three to five minutes. The clearing reaction is slower because they cannot rely on the driving energy of UV light. The changes in the molecules whether Silver halides or organic compounds are reversible and the molecules revert to their original form (equilibrium state) and the transparent state when the exposure is removed.
Clearing process – The darkening process is almost instantaneous. However, the lenses become clear in three to five minutes. The clearing reaction is slower because they cannot rely on the driving energy of UV light. The changes in the molecules whether Silver halides or organic compounds are reversible and the molecules revert to their original form (equilibrium state) and the transparent state when the exposure is removed.
Factors that affect the transition
i. UV exposure – More the exposure more will be the darkening
ii. Thickness – Thicker glass has a darker tint as compared to the thinner ones.
iii. Temperature – The darkening is inversely proportional to the temperature that is more the temperature less will be the darkening.
 Advantages and disadvantages
Advantages and disadvantages
These colour changing lenses provide the correct amount of protection for the varying light conditions and absorb 100% UV radiation that can otherwise damage the eyes.
However, they do not adjust immediately and take a few minutes to change from light to dark or vice versa. They don’t always work effectively in cars because windscreens naturally screen out most of the ultraviolet light and thus they do not acquire the tint.
https://www.youtube.com/watch?v=pnsVks0SshA
Now that you know the reason behind the magical Photochromic glasses, you can spread the knowledge amongst your friends who are unaware of it. So, stay curious and keep learning.
You will be actually a excellent web marketer. Your website loading rate is usually extraordinary Colour Blindness Treatment. It kind of feels that you’ll be undertaking any kind of one of a kind key. In addition, The subject matter will be must-see. you’ve got completed a wonderful activity during this make any difference!
Frequently I don’t find out post upon websites, even so wish to declare that that write-up extremely urged myself for you to do consequently! The composing preference may be amazed my family.. artifical eye to human Thank you, fairly wonderful post.
I have understand some great stuff in this article. Certainly price bookmarking intended for revisiting Color Blindness Treatment India. I personally amaze the amount of hard work you put to generate this kind of superb beneficial web site.
Howdy, Nice article artificial eye. There is an concern with your web-site around web ie, can go here? IE still is this market director along with a substantial component of individuals leaves out there great composing because of this issue.
Wonderful web site. A great deal of beneficial facts below. Now i’m delivering them to your associates ans as well discussing with delectable. As well as, thanks a lot in the perspiration!
An individual necessarily help to produce critically articles I would personally condition.. color blind engineer That’s the very first time that My spouse and i been to your internet site site and therefore considerably? I impressed with the examination you have made in making this actual post extraordinary. Great pastime!
Now i’m definitely influenced with the writing talents and as well with the structure in your site.. colour blindness symptoms Is that this any paid for issue or even does one customize it yourself? Regardless be on the superior quality creating, it is unusual to see an incredible website exactly like it nowadays.
I have been previously exploring on the net a lot more than several several hours as of late, however in no way discovered any kind of interesting write-up for instance you. It is really very price tag satisfactory in my opinion. In my opinion, if perhaps all web owners plus writers created good content since you likely have, the net might be a much bigger handy than before.
Excellent post! We will be linking to this particularly great content on our site.
Keep up the good writing.
Handy facts. Lucky me personally I discovered your web site by chance, with this particular gob smacked the reason why this kind of coincidence decided not to took place before hand! My spouse and i added them crutch glasses in uttar pradesh.
Your style is unique in comⲣarison to other folks I
have read stuff from. Many thanks for posting when you hаve the opportunity, Guesѕ I’ll just book mark this Ьloɡ.
Enormous elements listed here. I’m just extremely content to search ones content. Cheers with this particular looking ahead to contact you.. ocular prosthesis Can you generously drop us a e-mail?
Thanks for your expression of interest. Please share your inputs/suggestions on [email protected] for the relevant team members to look into it with all the details. You can give my reference.
It is a good plus helpful little bit of information color blind engineer. We’re joyful you shared this beneficial facts with us. Please continue being all of us up-to-date such as this. Appreciate sharing.
Fantastic beat! I need to novice whenever you amend your blog, how do i register for the blog website? Your consideration helped me a acceptable cope. I Artificial Eye suppliers am somewhat common on this your own over the air available vibrant see-thorugh notion
My buddy recommended I could in this way site. Your dog appeared to be fully perfect sun vitamin. This specific post in fact created my day time. You can’t visualize just simply how much time frame I did invested with this details! Appreciate it!
I enjoy, trigger I ran across what exactly I’m having a look for. You might have finished my personal four time very long look! Goodness Thanks a lot gentleman. Possess a great working day. L8rs