The number of electrons, protons and neutrons in a species are equal to 18, 16 and 16 respectively. Assign the proper symbol to the species.
The atomic number is equal to the number of protons = 16. The element is sulphur (S).
Mass No. = No. of protons + No. of neutrons
= 16 + 16 = 32.
Since the number of protons is not equal to the number of electrons, therefore the species is not neutral. It is anion (negatively charged) with charge equal to -2. Hence the symbol is 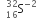 .
.
When is the number of protons and electron same in an atom?
Why are electrons called planetary electrons?
