What are half cell reactions? Explain.
An electrons cell is made up of two half cells in each of which a half-reaction takes place. The half reaction taking place in each half cell is known as half cell reaction. A half cell reaction in which electrons are released or oxidation occurs is called oxidation half cell reaction and the one in which electrons are accepted or reduction occurs is called reduction half-cell reaction, e.g. in Daniell cell, two half reactions taking place are:
(i) Oxidation half cell reaction (Anode reaction):
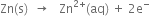
(ii) Reduction half cell reaction (Cathode reaction):
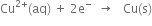
What do you mean by redox couple ?
A galvanic cell can always be represented by a cell diagram.
(i) The anode is written on the left-hand side and represented by writing metal first and then the metal ions (or electrolyte). The two are separated by a vertical line.
Zn | Zn2+ (1M)
Pt, H2(1 atm); H+(1M)
(ii) The cathode is written on the right-hand side and is represented by writing metal ions (or electrolyte) first and then metal (or solid phase). The two are separated by a vertical line.
Cu2+ (1M) | Cu
(iii) The salt bridge is represented by two vertical lines separating the two half cells. Daniell cell is represented as;
Zn | Zn2+ 1M) || Cu2+ (1M) | Cu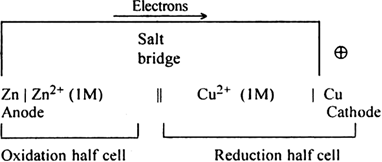
Write down the half reaction and net reaction for the Daniell cell.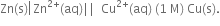
The half reaction of given cell is,
(i) Oxidation half-reaction:
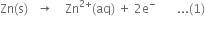
(ii) Reduction half-reaction:
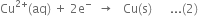
Net reaction {By adding (1) and (2)}
Electrochemical cell: A device employed to convert the chemical energy of a redox reaction into electrical energy is called an electrochemical cell. It is also called the galvanic or voltaic cell.
Main requirements:
(i) A suitable redox reaction is carried out indirectly in two separate half-cells. The electrons are lost in one-half cell and gained in the other half cell.
(ii) The substance which loses the electrons and the one which accepts the electrons should not be in direct contact with each other. The electron transfer must take place through an external circuit.
