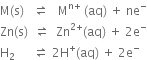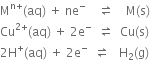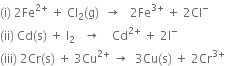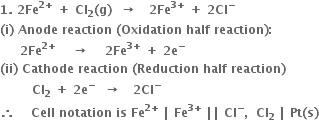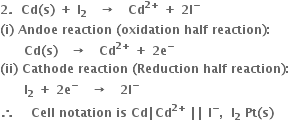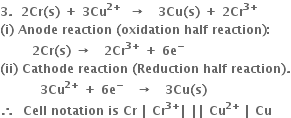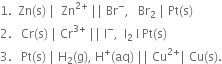
1. For the cell
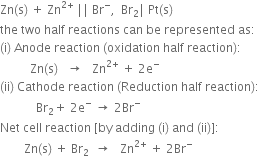
Positive terminal: The bromine electrode where reduction takes place.
2. For the cell 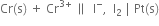
The two half reactions can be represented as:
(i) Anode reaction (oxidation half reaction):
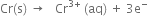
(ii) Cathode reaction (reduction half reaction):

Positive terminal. The iodine electrode where reduction takes place.
3. For cell 
the two half reactions can be represented as:
(i) Anode reaction (oxidation half reaction):

(ii) Cathode reaction (reduction half reaction):

Net cell reaction: It is obtained by adding equations (i) and (ii).
Positive terminal: The copper electrode where reduction takes place.
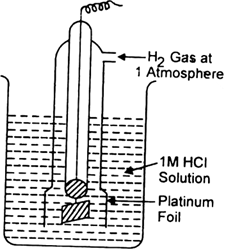
What is electrode potential? Name the factors on which it depends.