Under which major headings the following items will be presented in the Balance sheet of a company as per Schedule VI Part I of the Companies Act, 1956?
(i) Loans provided repayable on demand
(ii) Goodwill
(iii) Copyrights
(iv) Loose tools
(v) Cheques
(vi) General Reserve
(vii) Stock of finished goods and
(viii) 9% Debentures repayable after three years
| Items | Major Head | Major-Head | Sub-Head |
| (i) | Loans provided repayable on demand | Current Assets | Short-Term Loans and Advances |
| (ii) | Goodwill | Non-Current Assets | Fixed Assets - Intangible Assets |
| (iii) | Copyrights | Non-Current Assets | Fixed Assets- Intangible Assets |
| (iv) | Loose Tools | Current Assets | Inventories |
| (v) | Cheques | Current Assets | Cash and Cash Equivalent |
| (vi) | General Reserve | Shareholder's Funds | Reserves and Surplus |
| (vii) | Stock of Finished Goods | Current Assets | Inventories |
| (viii) | 9% Debentures repayable after three years | Non-Current Liabilities | Long-Term Borrowings |
Under which heads and sub-heads the following items will appear in the Balance Sheet of a company as per revised Schedule VI, Part-I of the Companies Act 1956.
i. Premium on Redemption of Debentures
ii. Loose Tools
iii. Balance with Banks
| Items | Heads | Sub-Heads |
| Premium on Redemption of Debentures | Non- Current Liabilities | Other Long-Term Liabilities |
| Loose Tools | Current Assets | Inventories |
| Balance with Banks | Current Assets | Cash and Cash Equivalents |
Under which major sub-headings the following items will be placed in the Balance Sheet of a company as per revised Schedule-VI, Part-I of the Companies Act, 1956:
(i) Accrued Incomes
(ii) Loose Tools
(iii) Provision for employees benefits
(iv) Unpaid dividend
(v) Short-term loans
(vi) Long-term loans.
|
ITEMS |
SUB-HEAD |
|
(i) Accrued Incomes |
Other Current Assets |
|
(ii) Loose Tools |
Inventories |
|
(iii) Provision for Employees benefits |
Short-Term Provisions |
|
(iv) Unpaid Dividend |
Other Current Liabilities |
|
(v) Short-Term Loans |
Short-Term Borrowings |
|
(vi) Long-Term Loans |
Long-Term Borrowings |
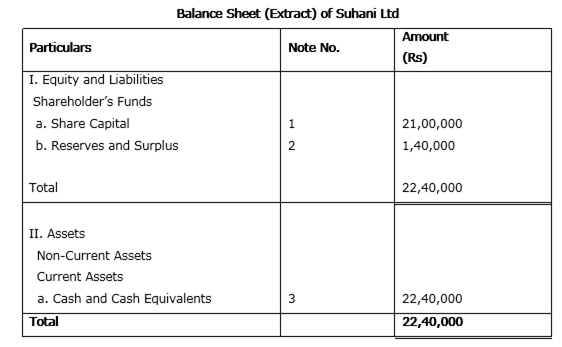
The authorized capital of Suhani Ltd. is Rs. 45,00,000 divided into 30,000 shares of Rs. 150 each. Out of these company issued 15,000 shares of Rs. 150 each at a premium of Rs. 10 per share.
The amount was payable as follows:
Rs. 50 per share on application, Rs 40 per share on allotment (including premium), Rs. 30 per share on first call and balance on final call. Public applied for 14,000 shares. All the money was duly received.
Prepare an extract of Balance Sheet of Suhani Ltd. as per Revised Schedule VI Part I of the Companies Act 1956 disclosing the above information. Also prepare notes to accounts for the same.

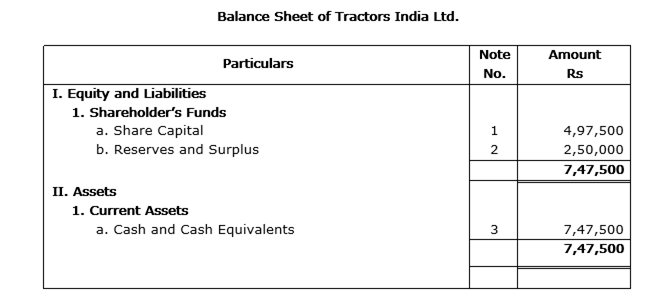
'Tractors India Ltd.' is registered with an authorized capital of Rs 10,00,000 divided into 1,00,000 equity shares of Rs 10 each. The company issued 50,000 equity shares at a premium of Rs 5 per share. Rs 2 per share were payable with application, Rs 8 per share including premium on allotment and the balance amount on first and final call. The issue was fully subscribed and all the amount due was received except the first and final call money on 500 shares allotted to Balaram.
Present the 'Share Capital' in the Balance Sheet of 'Tractors India Ltd.' as per Schedule VI Part I of the Companies Act, 1956. Also prepare Notes to Accounts for the same.