Classify the following transformations according to the reaction type.![]()
In this reaction, substitution takes place, followed by a rearrangment of atoms and groups of atoms.
Why carbanions are very negative? Discuss the structure of carbanion.

Give the hybridisation state of each carbon in the following species: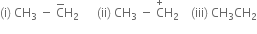
The hybridisation of the given compound :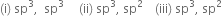
Give reasons why the following two structures I and II cannot be the major contributors to the real structure of CH3COOCH3: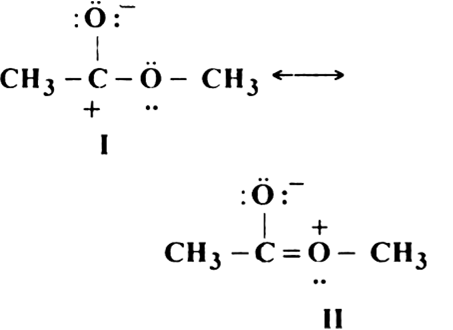
The two structures are less important contributors as they contain charge separation. Also, the structure I contain a carbon atom with an incomplete octet.
Give two methods for the generation of carbocation. Describe its structure.
(i) Direct ionisation: The carbon-halogen bond (C-X) in many organic halides generates carbocations in the presence of a highly polar medium.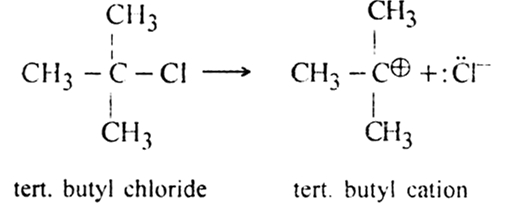
(ii) Protonation of alkene:![]()
The structure of carbocation: The positively charged carbon of the carbocation is sp2 state of hybridisation. The three sp2 hybridised orbitals which lie in the same plane are involved in the formation of three bonds with other atoms. The unhybridised p-orbital remains vacant. Thus, the carbocation has a flat structure. 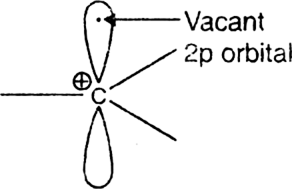
The bond angle around the positively charged carbon is nearly 120° each.
