What do you mean by:
(i) Elimination reactions
(ii) Rearrangement reactions?
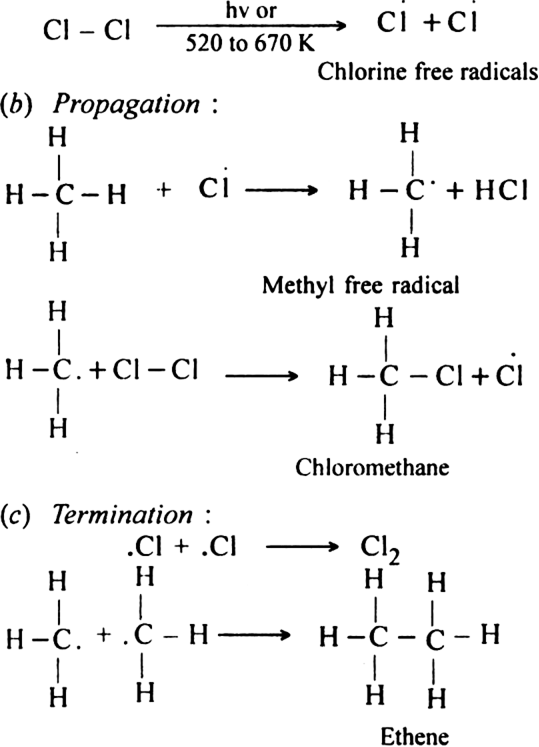
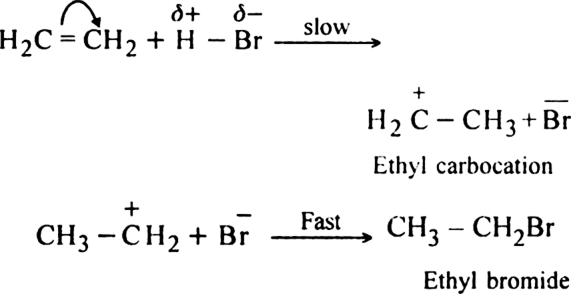
(i) Elimination reactions: An elimination reaction is one which involves the loss of two atoms or groups either from the same or adjacent atoms of a substance leading to the formation of multiple bond i.e. double or triple bond. In such reactions at least two σ; bonds are lost and one 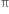 ; bond is created. These are two types:
; bond is created. These are two types:
(a) β-elimination reaction. Here loss of two atoms or groups takes place from the adjacent carbon atoms in the molecule. For example,
(i) Dehydrohalogenation of alkyl halides with an alcoholic solution of KOH.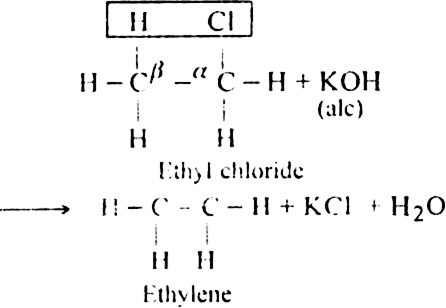
(ii) Dehydration of alcohols in the presence of concentrated H2SO4 upon heating: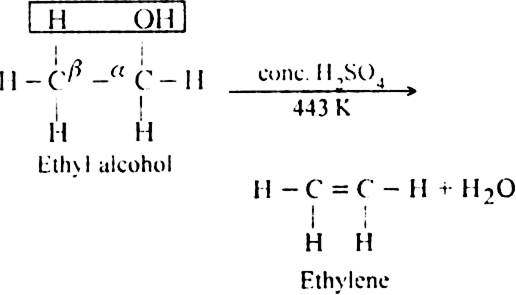
(iii)  -Elimination reactions. In such reactions, there is loss or elimination of two atoms or groups from the same carbon atom in the molecule. For example.
-Elimination reactions. In such reactions, there is loss or elimination of two atoms or groups from the same carbon atom in the molecule. For example.
(b) Dehydrohalogenation of chloroform with NaOH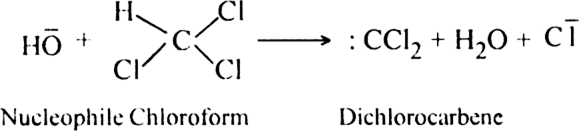
(ii) Dehydrogenation of primary or secondary alcohols with reduced copper at 573K.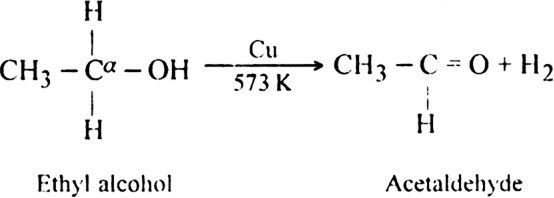
(iii) Rearrangement reactions Reactions involving the shift or migration of an atom or a group from one atom to another within the same molecule are called rearrangement reactions for example.
(i) n-Butane rearranges to form 2-methyl propane when heated in a sealed tube in the presence of AlCl3/HCl.
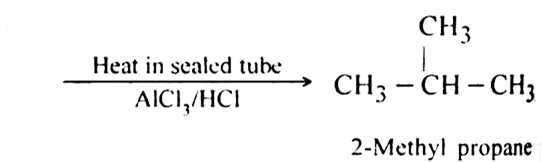
(ii) Ammonium cyanate upon heating rearranges to form urea.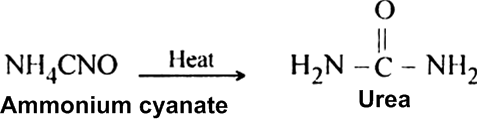
What do you understand by:
(i) Isomerisation reaction
(ii) Condensation reaction?
(i) Isomerisation reaction. All those reactions in which interconversion of isomers take place without affecting the molecular formulae and carbon skeletons of reactants and products are called Isomerisation reactions. For example,
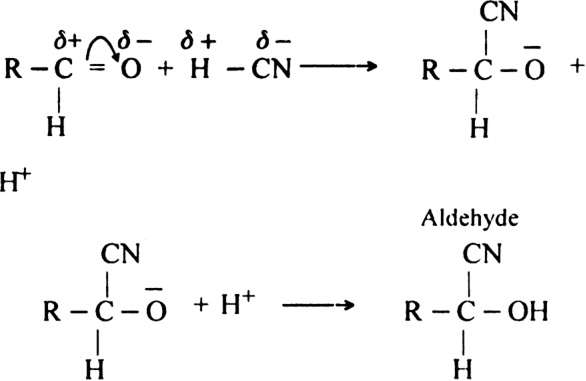
(ii) Condensation reaction. All those reactions in which two same or different organic reactants unite to give a product with or without the elimination of another, simple molecule are called condensation reaction. For example condensation of aldehyde or ketone.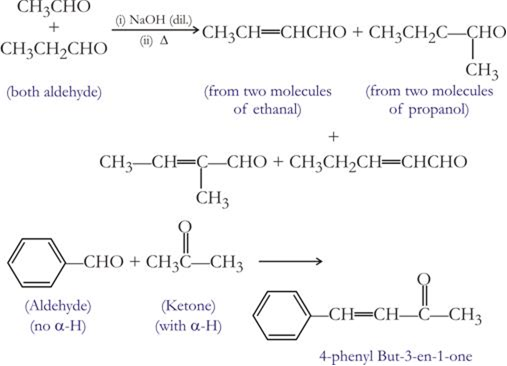
What do you understand by:
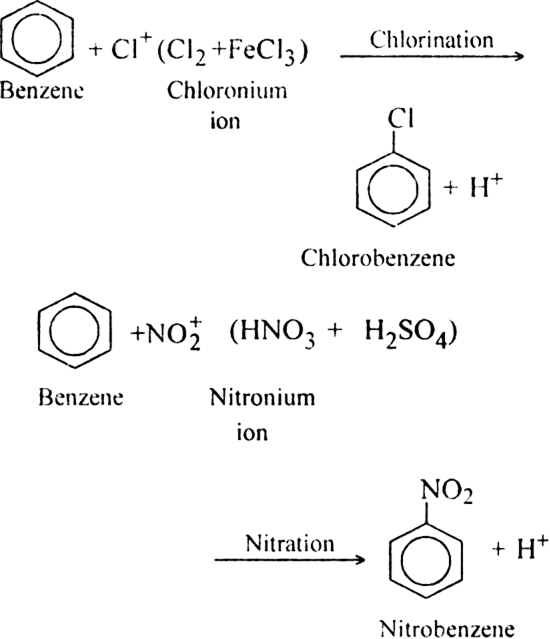
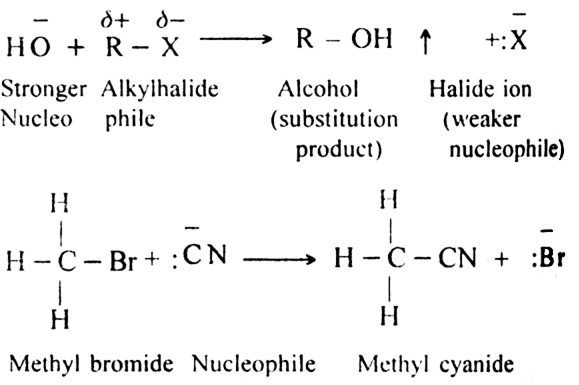
(i) Substitution reactions
(ii) Addition reactions?

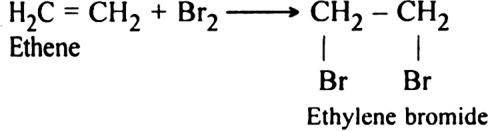
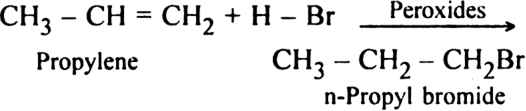

How will you effect the separation of a mixture of acetone and methyl alcohol which are miscible with each other?
Or
Give a brief description of fractional distillation.
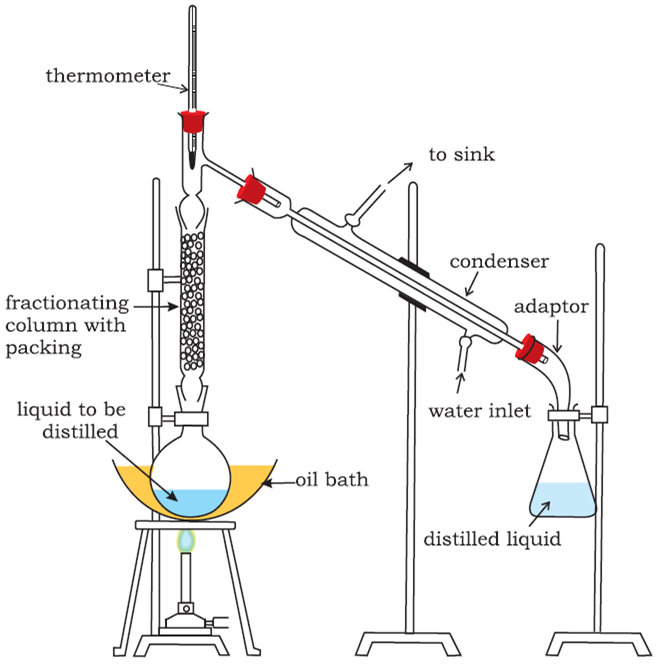
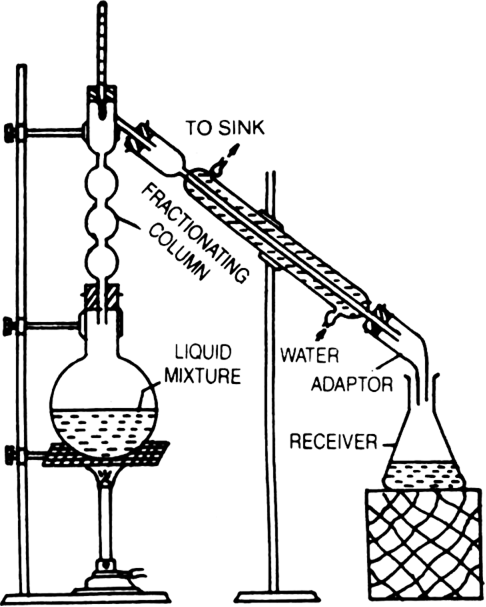
Fractional distillation: This process is generally used for those mixtures, where the difference in the boiling points of the two liquids is less than 15 K. Acetone boils at 330 K while methyl alcohol at 338 K, therefore, these two liquids are separated by the fractional distillation process. This is similar to the ordinary distillation method with the only exception that a fractionating column is introduced in between the distillation flask and the condenser.
The process of separation of the components of a liquid mixture at their respective boiling points in the form of vapours and the subsequent condensation of these vapours is called fractional distillation.
The fractionating columns used for the purpose are of different shapes as shown in the figure.