Explain the terms:
(i) Electrophiles
(ii) Nucleophiles
Or
What are electrophiles and nucleophiles? Explain with examples.
 (Nitronium ion),
(Nitronium ion),
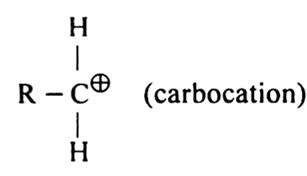
What do you understand by:
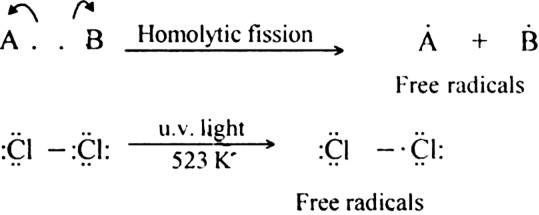
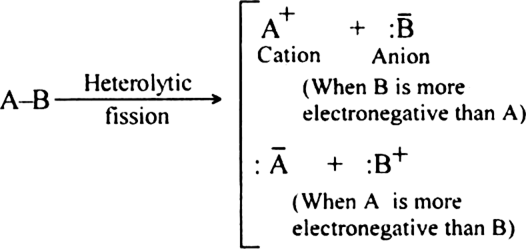
(i) Homolytic fission
(ii) Heterolytic fission?
Give the IUPAC names of the following compounds: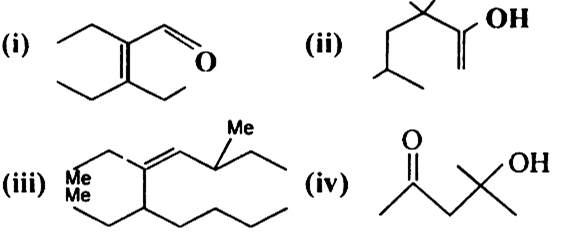
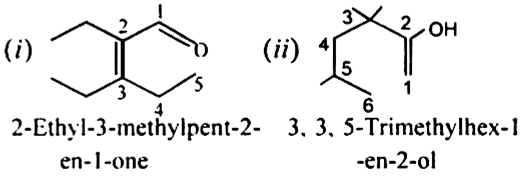
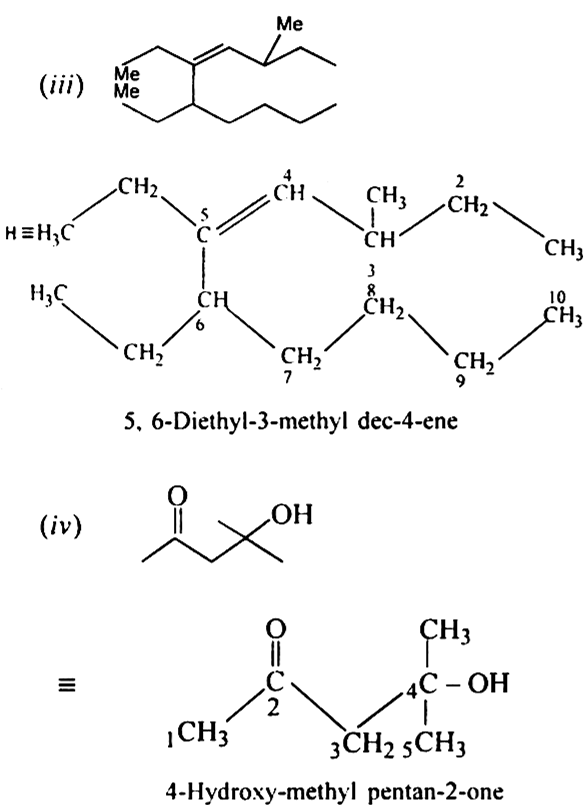
Give the IUPAC names of the following compounds: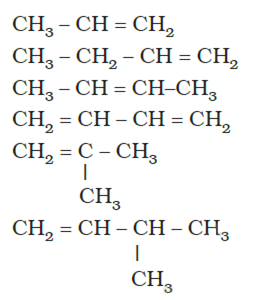
i)Propene
ii) But-1-ene
iii) But-2-ene
iv) Buta-1,3-diene
v) 2-methylprop-1-ene
vi) 3-methylprop1-ene
vii) 3-methylbut-1-ene
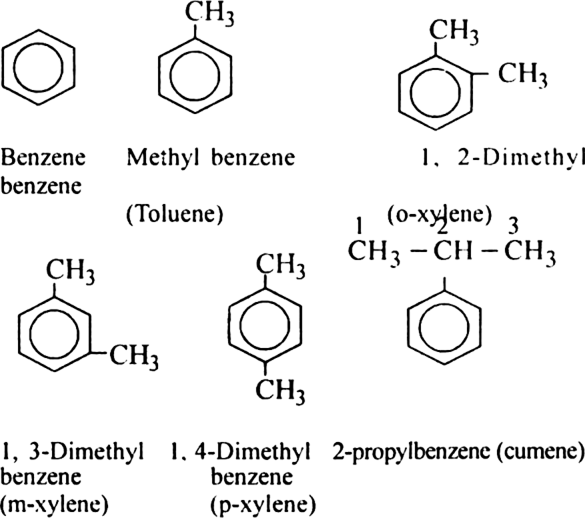
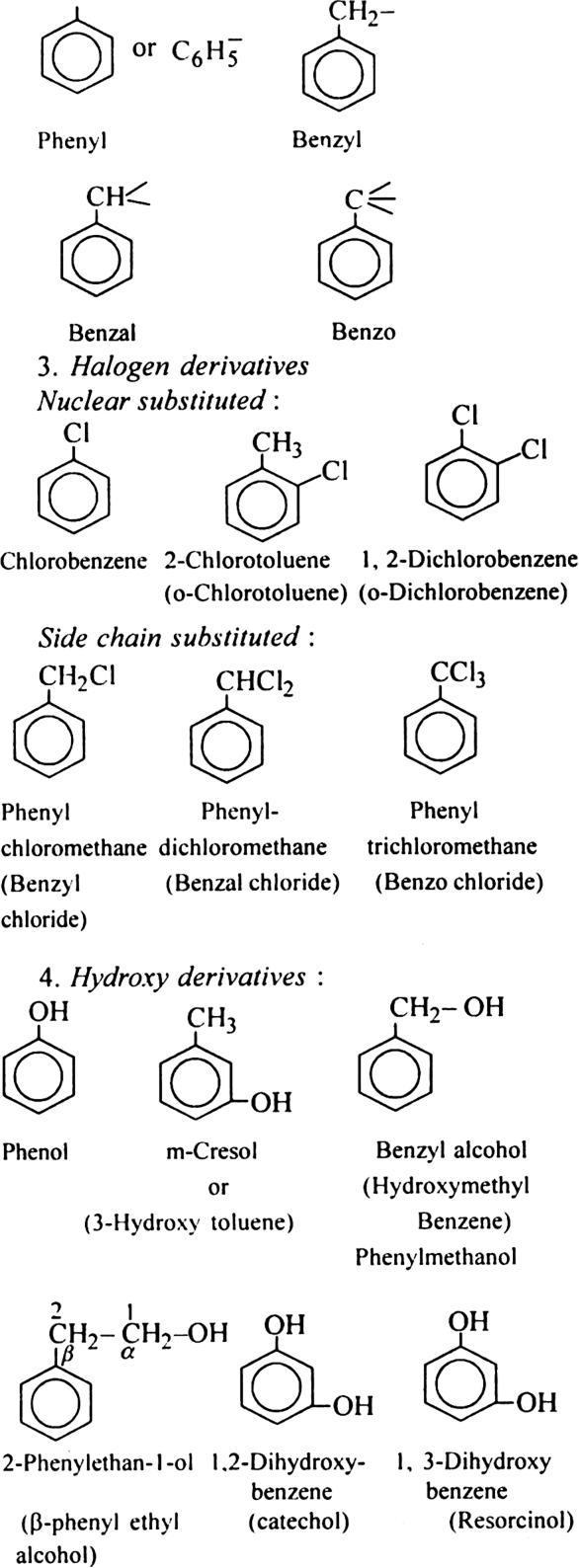
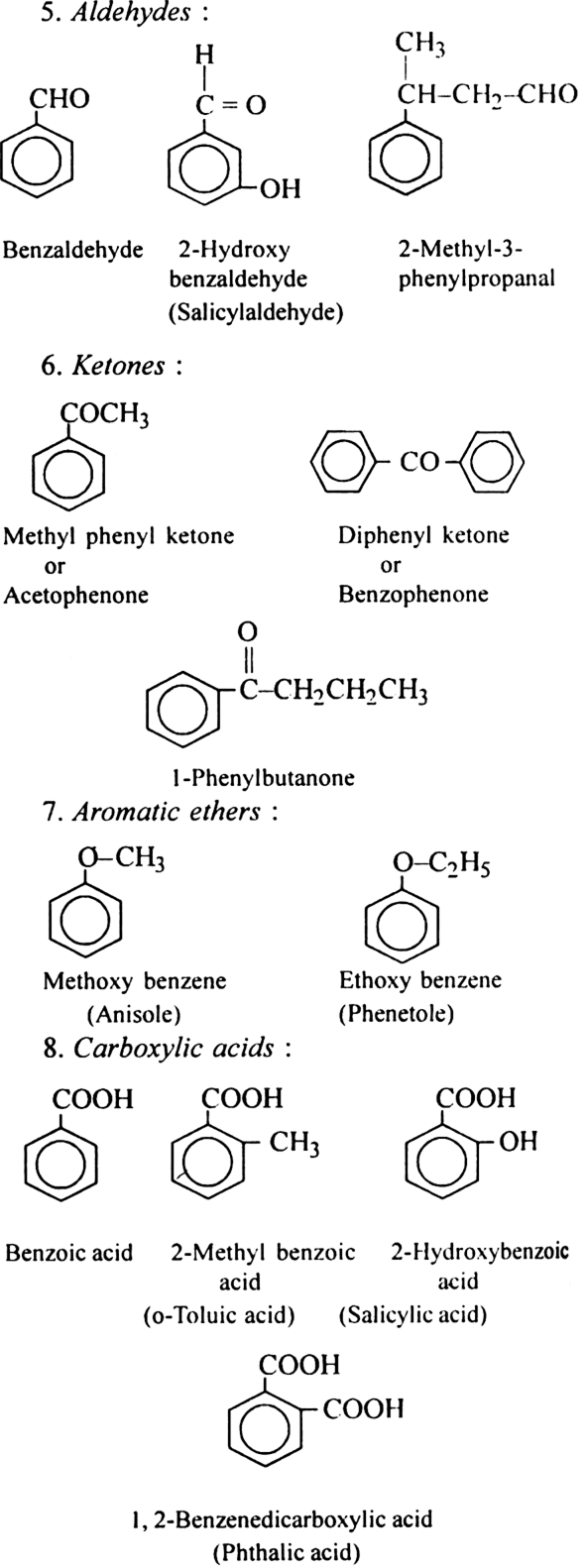
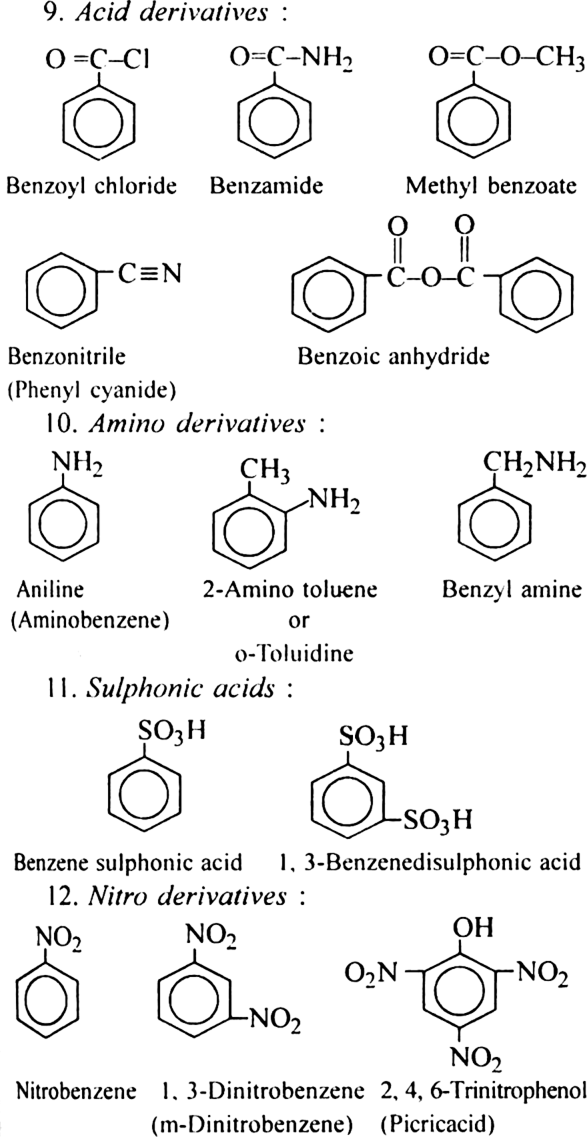
Discuss the nomenclature of simple aromatic compounds.
Aromatic compounds contain one or more isolated or fused benzene rings. Aromatic compound consists of two parts:
(i) nucleus (ii) side chain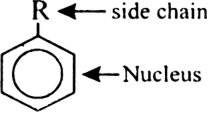
(i) Nuclear substituted compounds are those in which the functional group is directly attached to the benzene ring. In the IUPAC system, they are named as derivatives of benzene. The position of the substituents in disubstituted benzene are indicated as 1,2; 1, 3 and 1, 4. These are respectively called ortho (or o-), meta (or m–) and para (or p-).
(ii) Side chain substituted compounds are those in which the functional group is present in the side chain of the benzene ring. Both in the common and IUPAC system, these are usually named as phenyl derivatives of the corresponding aliphatic compounds (except arenes which are named as derivatives of benzene in the IUPAC system). The position of the substituents on the side chain including the benzene ring are indicated as α, β, γ....in the common system and I, 2, 3 etc. in the IUPAC system.
1. Aromatic hydrocarbons (Arenes). Hydrocarbons which contain both aliphatic and aromatic units are called arenes.