Discuss briefly the anomalous behviour of elements of second period.
Or
The first element of each group differs significantly from rest of the elements of the same group. Give reasons.
The first element of each of the groups 1 (lithium) and 2 (beryllium) and groups 13-17 (boron to fluorine) differs in many respects from the other members of their respective group. The difference in the behaviour of the first member of a group in the s- and p-block compared to the other members in the same group can be due to the following factors:
(i) Small atomic size of the first element.
(ii) Large charge/radius ratio
(iii) High electronegativity
(iv) The absence of d-orbitals in the valence shell of the first element.
The first member of s- and p-block has only four, valence orbitals (2s and 2p) available for bonding. As a result, the maximum covalency of the first member of each group is 4. On the other hand, the second member of the groups has nine valence orbitals (3s, 3p, 3d). Hence these members can expand their covalency beyond 4, through the participation of d-orbital in bond formation.
(v) Ability to form pπ-pπ multiple bonds. The first member of each group of p-block elements has great tendency to form pπ-pπ multiple bonds to itself (C = C. C ≡ N, N = N, N ≡ N and to the other second period N, N ≡ N) elements (C = 0, C = N, C ≡ N, N = 0).
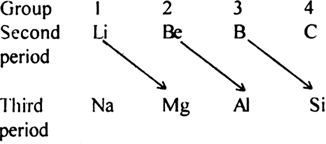
What do you understand by diagonal relationship?
The valence of representative elements is either equal to the number of valence electrons or eight minus the number. What is the basis of their rule?
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 |
| Compounds | LiH | BeH2 | BH3 | CH4 | NH3 | H2O | HF |
| No. of valence electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Valency of element | 1 | 2 | 3 | 4 | 8-5 =3 | 8-6 =2 | 8-7 =1 |
The valency of any element is the number of electrons which it loses, joins or shares with other atoms to attain the nearest inert gas configuration. The elements of groups 1, 2 and 13 lose 1, 2 and 3 electrons respectively to attain the nearest noble gas configuration. Thus, the valency of the elements of groups 1, 2 and 13 is equal to the number of their valence electrons. The elements of group 14 have four electrons in their respective valence shells and thus attain noble gas configuration only by sharing with four more electrons of other atoms. Therefore, the valency of elements of group 14 is also equal to the number of their valence electrons.
The elements of group 15, 16 and 17 have 5, 6 and 7 electrons in their respective valence shells and hence they acquire 3 (8-5), 2 (8-6) and 1 (8-7) electrons either by gaining or sharing electrons from other atoms to attain the nearest inert gas configuration. Thus, the valency of the elements of groups 15, 16 and 17 is equal to eight minus the number of electrons in their respective valence shells.
