What weight of Ca will contain the same number of atoms as are present in 3.2g of sulphur?
Atomic mass of sulphur = 32 amu
Gram atomic mass of sulphur = 32 g
1 mole of sulphur contains = 6.023 x 1023 atoms
Now 32 g of sulphur contains
= 6.023 x 1023 atoms
3.2 g of sulphur would contain
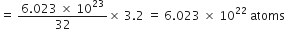
Gram atomic mass of calcium = 40 g
1 mole of calcium = 40 g
= 6.023 x 1023 atoms
Now 6.023 x 1023 atoms of calcium have mass = 40 g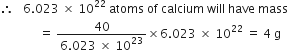
Define the term significant figures.
Significant figures are meaningful digits which are known with certainty.
