The standard reduction potential of an electrode is determined by connecting it with a standard hydrogen electrode whose electrode potential is taken as zero.
Calculation of E° of zinc. A cell consisting of zinc electrode immersed in 1 M ZnSO4 solution and [Standard hydrogen electrode is set up as shown.
The reading as given by voltmeter in the cell circuit is 0.76 V. This measures the E.M.F. of the cell. The electrons flow from zinc electrode to the hydrogen electrode. This means that zinc acts as an anode while hydrogen gas electrode acts as a cathode.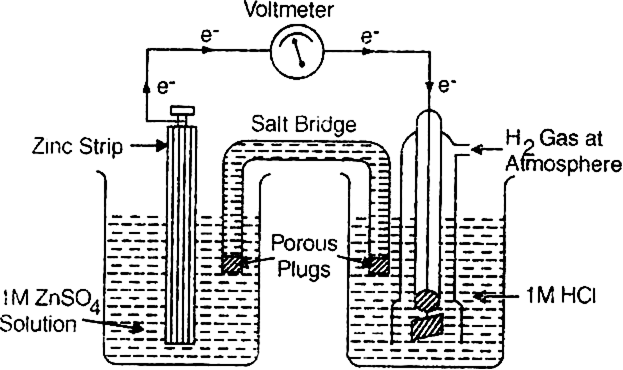
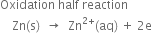
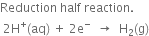
Since oxidation occurs at the zinc electrode, therefore, the standard electrode potential forZn2+|Zn half cell is –0.76 volt.
Calculation of E° of copper. A cell consisting of copper electrode immersed in 1M CuSO4 solution and standard hydrogen electrode is set up as shown.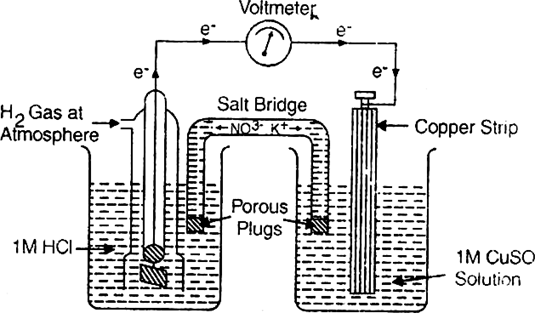
The reading as given by voltmeter in the cell circuit is 0.34V. This measures the E.M.F. of the cell. The electrons flow from hydrogen electrode to copper electrode. This means that hydrogen electrode acts as an anode while copper electrode acts as a cathode.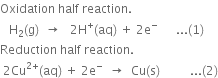
Since reduction occurs at the copper electrode, therefore, the standard electrode potential for Cu2+|Cu half cell is +0.34 volt.
Applications:
(i) To predict the relative oxidising and reducing powers: Greater the reduction potential, more easily the substance is reduced and hence is a stronger oxidising agent. For example oxidising powers of halogens are F2 > Cl2 > Br2 > I2.
(ii) To predict whether a metal will react with acid to give H2 gas : Metals above hydrogen in the series displace hydrogen from acids.
(iii) To calculate the standard E.M.F. of the cell
(iv) To predict the spontaneity of any redox reaction: If E.M.F. of the cell is positive, it is spontaneous, otherwise not.
What is reduction (electronic concept)?
We know zinc has negative reduction potential [E° (Zn2+1 Zn) = –0 . 76V] and lies above hydrogen in the electrochemical series. Therefore, the electron accepting tendency of zinc is less than that of hydrogen or its electron releasing tendency is more.
Thus, zinc can lose electrons to H+ ions of the acid and as a result, hydrogen gas is liberated.
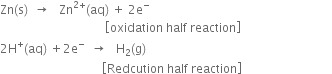
Since copper has a positive reduction potential (E° = +0.34 V) and lies below hydrogen in the electrochemical series, therefore, it cannot lose an electron to H+ ions of the acid. Hence H2 gas is not liberated.
