
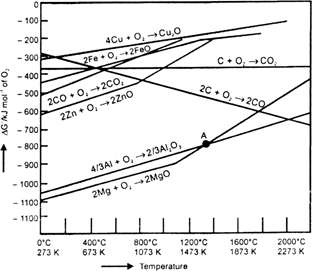
Is it true that under certain conditions. Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
Which of the ores mentioned in Table 6.1. could be concentrated by magnetic separation method?
Table 6.1 Principal ores of some important metal.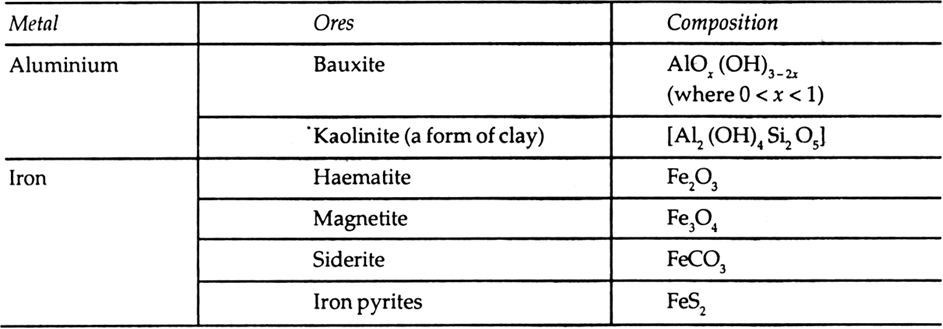

The role of depressant in froth floatation process is to prevent certain type of materials from forming the froth with bubbles. For example, to separate two sulphide ores (ZnS and Pbs), NaCN is used as a depressant which selectively allows PbS to come with froth, but prevents ZnS from coming to froth. This happens because NaCN reacts with ZnS to form Na2[Zn(CN)4].
4NaCN+ZnS → Na2[Zn(CN)4] + Na2S
What is the significance of leaching in the extraction of aluminium?
The reaction.
Cr2O3 + 2Al → Al2O3 + 2Cr (ΔG° = – 421 kJ)
is thermodyanmically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
At room temperature, all reactants and products of the given reaction are in the solid state. As a result, equilibrium does not exist between the reactants and the products. Hence, the reaction does not take place at room temperature.Certain amount of activation energy is essential even for such reactions which are thermodynamically feasible.
