What are the changes caused due to force applied on an object?
Describe an activity to observe the relation of pressure and surface area of an object.
Relationship between pressure and surface area of the object can be illustrated using the following activity.
Materials Required:
A soft bulletin board, chart paper and drawing pins.
Procedure:
1. Pin the chart paper on a soft bulletin board with flat rounded end of the drawing pin.
2. Now try pinning the chart papers with the sharp pointed ends of the drawing pin.
Observations:
In the first case, when the rounded end of the pin was used, only an impression of the pin formed on the chart paper.
In the second case, the sharp end makes a hole in the chart paper and hence can be fixed on the bulletin board.
Conclusion:
The surface area of the rounded end of the drawing pin is larger than the surface area of the sharp end. Therefore, a greater pressure is produced with the sharp end of the drawing pin.
This proves that, Pressure = Force per unit Area.
An inflated gas balloon is placed in a sealed jar which is connected to an evacuating pump. What will be observed if the air inside the jar is pumped out? Give a reason justifying your answer.
When air is pumped into the balloon from the sealed jar, the balloon will expand rapidly and burst. Balloon contains gas whose pressure which is more than the atmospheric pressure. This atmospheric pressure opposes the pressure of the gas inside the balloon.
If the air in the jar is pumped out, the pressure exerted by the gas in the balloon will not be opposed. Therefore, the balloon expands rapidly and bursts.
How can you demonstrate the presence of atmospheric pressure experimentally? Illustrate with a diagram.
Objective:
To demonstrate the presence of Atmospheric Pressure
Procedure:
1. We take a metallic can and add a little water in it.
2. We then remove its lid/ cap and heat the can, as shown in Fig.A.
3. The water boils and steam starts coming out from the mouth of the can.
4. The steam forces out most of the air from the can.
5. Now the can is closed with an air tight lid.
6. If we now pour cold water on the can, we observe that the can crushes.
Explanation:
When the can is heated and then capped, the hot air is filled inside the can.
Due to the cold water, the steam inside the can condenses to liquid state. Thus, a partial vacuum is created. In this case, the external atmospheric pressure becomes greater than the inside pressure. Hence high external pressure crushes the container.
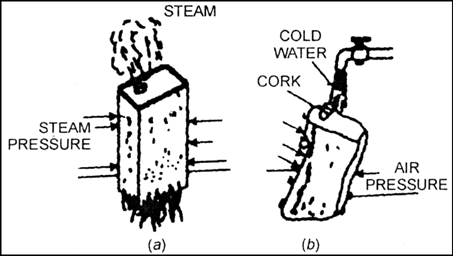
Fig-A
What are the contact forces? Describe two forces that are contact forces.
The force acting on objects, when two objects come in contact with each other are known as contact forces.
Examples of contact forces are given below:
1. Muscular Force: Force exerted due to the action of muscles. This force is categorised as contact force because, it comes in play only when an object is being handled.
Example: bending, running, moving etc.
2. Friction: Force exerted on an object when its surface comes in contact with another surface. This force comes into action only when the two surfaces come in contact with each other and hence are called contact forces.
