
What are polyatomic ions? Give examples.
A group of atoms carrying a charge is known as a
polyatomic ion. For example, hydroxide ion (OH–), carbonate ion (CO3–2).
Calculate the formula unit masses of ZnO, 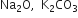 , given atomic masses of Zn = 65 u. Na = 23 u, K = 39 u, C = 12 u, and O = 16 u.
, given atomic masses of Zn = 65 u. Na = 23 u, K = 39 u, C = 12 u, and O = 16 u.
Formula unit mass of ZnO = Atomic mass of Zn + Atomic mass of O = 65 + 16 = 81 u
Formula unit mass of Na2O = 2 × Atomic mass of Na + Atomic mass of O = 2 × 23 + 16 = 62u
Formula unit mass of K2CO3 = 2 × Atomic mass of K + Atomic mass of C + 3 × Atomic mass of O = 2 × 39 + 12 + 3 × 16 = 138u.
A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
We have given,
Mass of boron = 0.096g
Mass of oxygen = 0.144g
Mass of sample =0.24g
Thus, percentage of boron by weight in the compound =
Thus, percentage of oxygen by weight in the compound =
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
Chemical formulae of the following is:
(a) Magnesium chloride – MgCl2
(b) Calcium oxide – CaO
(c) Copper nitrate – Cu(NO3)2
(d) Aluminium chloride – AlCl3
(e) Calcium carbonate – CaCO3
When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen ? Which law of chemical combination will govern your answer?
When 3.0 g carbon is burnt in 8.00 g oxygen, 11.00 g carbon dioxide is formed. It means all of carbon and oxygen are used up and carbon and oxygen are combined in the ratio of 3 : 8 to form carbon dioxide. Thus when there is 3 g carbon and 50.0 g oxygen, then also only 8 g oxygen will be used and 11.0 g carbon dioxide will be formed. The remaining oxygen is not used us. This indicates law of definite proportions which says that in compounds, the combining elements are present in definite proportions by mass.
