 Multiple Choice Questions
Multiple Choice QuestionsThe reaction of propene with HOCl(Cl2+H2O) proceeds through the intermediate:
CH3−CH+−CH2−Cl
CH3−CH(OH)−C+H2
CH3−CHCl−C+H2
CH3−CHCl−C+H2
A.
CH3−CH+−CH2−Cl
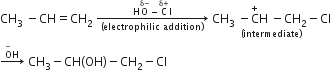
The synthesis of alkyl fluorides is best accomplished by
free radical fluorination
Sandmeyer's reaction
Finkelstein reaction
Finkelstein reaction
In SN2 reactions, the correct order of reactivity for the following compounds CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is
CH3Cl > (CH3)2CHCl >CH3CH2Cl > (CH3)3CCl
CH3Cl > CH3CH2Cl > (CH3)2CHCl >(CH3)3CCl
CH3CH2Cl > CH3Cl >(CH3)2CHCl >(CH3)3CCl
CH3CH2Cl > CH3Cl >(CH3)2CHCl >(CH3)3CCl
The major organic compound formed by the reaction of 1,1,1-trichloroethane with silver powder is
acetylene
ethene
2-butyne
2-butyne
The order of stability of the following carbocations,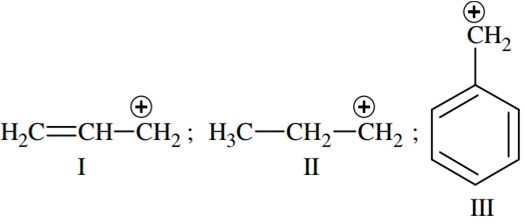
III>II>I
II> III>I
I>II>III
I>II>III
An unknown alcohol is treated with the “Lucas reagent” to determine whether the alcohol is primary, secondary or tertiary. Which alcohol reacts fastest and by what mechanism
Secondary alcohol by SN1
Tertiary alcohol by SN1
Secondary alcohol by SN2
Secondary alcohol by SN2
What is DDT among the following
Greenhouse gas
A fertilizer
Biodegradable pollutant
Biodegradable pollutant
In the given transformation, which of the following is the most appropriate reagent?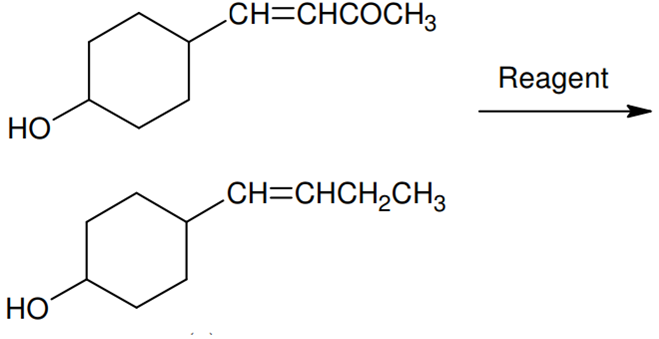
NH2NH2,O-H
Zn − Hg/HCl
Na,Liq.NH3
Na,Liq.NH3
