 Multiple Choice Questions
Multiple Choice QuestionsIdentify the correct method for the synthesis of the compound shown below from the following alternatives.
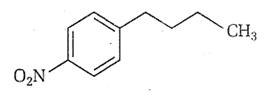
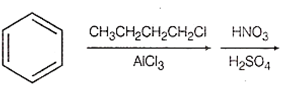
![]()
![]()
![]()
Which one of the following methods are used to prepare Me3COEt with good yield?
Mixing EtONa with Me2CCl
Mixing Me2CONa with EtCl
Heating a mixture of (1:1) EtOH and Me2COH in the presence of conc. H2SO4
Treatment of Me3COH with EtMgI
Under identical conditions, the SN1 reaction will occur most efficiently with:
tert-butyl chloride
1-chlorobutane
2-methyl-1-chloropropane
2-chlorobutane
Identify the method by which Me3CCO2H can be prepared:
Treating 1 mole of MeCOMe with 2 moles of MeMgl
Treating 1 mole of MeCO3Me with 3 moles of MeMgI
Treating 1 mole of MeCHO with 3 moles of MeMgl
Treating 1 mole of dry ice with 1 mol of MeCMgI
The correct statement regarding the following compounds is
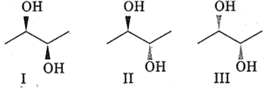
all three compounds are chiral
only I and II are chiral
I and III are diastereomers
only I and III are chiral
The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is:
3° < 2° < 1°
3° > 2° > 1 °
3° < 2° > 1°
3° > 2° < 1 °
When AgCl is treated with KCN
Ag is precipitated
a complex ion is formed
double decomposition takes place
no reaction takes place
Which one of the following compounds is most reactive towards nucleophilic addition?
CH3CHO
PhCOCH3
PhCOPh
CH3COCH3
A.
CH3CHO
Carbonyl compounds undergoes nucleophilic addition reaction.
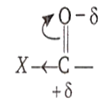
[X- shows negative inductive effect]
If group or atom attached with carbonyl carbon shows negative inductive effect, then it decreases electron density on carbonyl carbon and facilitate the attack of nucleophiles, hence reactivity of carbonyl compound increases.
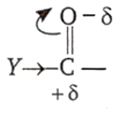
[Y- shows negative inductive effect]
If group or atom attached with carbonyl carbon shows positive inductive effect, then it increases electron density on carbonyl carbon and supress the attack of nucleophiles, hence, reactivity of carbonyl compound decreases.
The aromatic aldehydes and ketones are less reactive than their aliphatic analogues. This is due to the +R effect of benzene ring.
On the basis of above informations, the increasing order of the nucleophilic addition reaction in the following compound will be CH3CHO > CH3COCH3 > PhCOCH3 > PhCOPh
Which reaction is used for the preparation of acetophenone ?
Reimer-Tiemann reaction
Wurtz-Fittig reaction
Friedel-Craft's reaction
Cannizaro's reaction
