 Multiple Choice Questions
Multiple Choice QuestionsThe solubility product of silver bromide is 5.0 x 10-13. The quantity of potassium bromide (molar mass taken as 120 g mol–1)to be added to 1 litre of 0.05 M solution of silver nitrate to start the precipitation of AgBr is
1.2 x 10-10 g
1.2 x 10-9 g
6.2 x 10-5 g
6.2 x 10-5 g
For a particular reversible reaction at temperature T, ΔH and ΔS were found to be both +ve. If Te is the temperature at equilibrium, the reaction would be spontaneous when
Te>T
T >Te
Te is 5 times T
Te is 5 times T
Given
C(grahite) + O2(g) → CO2(g)
ΔrH° = - 393.5 kJ mol-1
H2(g) + 1/2O2(g) → H2O (l)
ΔrH° = +890.3 kJ mol-1
Based on the above thermochemical equations, the value of ΔrH° at 298 K for the reaction
C(grahite) + 2H2(g) →CH4 will be
+74.8 kJ mol–1
+144.0 kJ mol–1
–74.8 kJ mol–1
–74.8 kJ mol–1
In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is
CH3OH(l) + 3/2O2(g) → CO2(g) + 2H2O(l)
At 298 K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and CO2(g) are –166.2, –237.2 and –394.4 kJ mol–1 respectively. If
standard enthalpy of combustion of methanol is –726 kJ mol–1, efficiency of the fuel cell will be:
80%
97%
87%
87%
Which one of the following reactions of Xenon compounds is not feasible?
XeO3+ 6HF → XeF6 + 3H2O
3XeF4 +6H2O → Xe+XeO3+ 12HF+ 1.5O2
2XeF2 + 2H2O → 2Xe + 4HF +O2
2XeF2 + 2H2O → 2Xe + 4HF +O2
Oxidising power of chlorine in aqueous solution can be determined by the parameters indicated below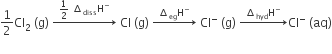
The energy involved in conversion of 
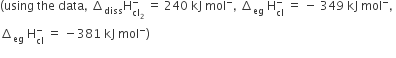
152 kJ mol-
-610 kJ mol-
-850 kJ mol-
-850 kJ mol-
Standard entropy of X2, Y2 and XY3 are 60, 40 and 50 JK−1
mol −1, respectively. For the reaction,1/2X2 + 3/2Y2, ΔH = -30 kJ,to be at equilibrium, the temperature will be
1250 K
500 K
750 K
750 K
In the reaction,
2Al(s) + 6HCl(aq) → 2Al3+ (aq) + 6Cl¯(aq) + 3H2(g)
6L HCl(aq) is consumed for every 3L H2(g) produced
33.6 L H2(g) is produced regardless of temperature and pressure for every mole Al that reacts
67.2 L H2(g) at STP is produced for every mole Al that reacts
67.2 L H2(g) at STP is produced for every mole Al that reacts
Identify the correct statement regarding a spontaneous process –
For a spontaneous process in an isolated system, the change in entropy is positive
Endothermic processes are never spontaneous
Exothermic processes are always spontaneous
Exothermic processes are always spontaneous
