 Multiple Choice Questions
Multiple Choice QuestionsPhenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid,gives
2,4,6-trinitrobenzene
o-nitrophenol
p-nitrophenol
p-nitrophenol
The reaction of toluene with Cl2 in presence of FeCl3 gives predominantly
benzoyl chloride
benzyl chloride
o-and p-chlorotoluene
o-and p-chlorotoluene
In the following sequence of reactions, 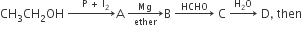 compound ‘D’ is
compound ‘D’ is
butanal
n-butyl alcohol
n-propyl alcohol
n-propyl alcohol
Fluorobenzene (C6H5F) can be synthesized in the laboratory
by heating phenol with HF and KF
from aniline by diazotisation followed by heating the diazonium salt with HBF4
by direct fluorination of benzene with F2 gas
by direct fluorination of benzene with F2 gas
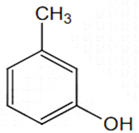
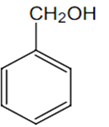
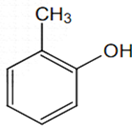
The structure of the compound that gives a tribromo derivative on treatment with bromine water is

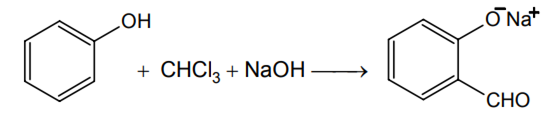
dichloromethyl cation (C+HCl )
dichlorocarbene ( :CCl2)
trichloromethyl anion (C-Cl3 )
trichloromethyl anion (C-Cl3 )
B.
dichlorocarbene ( :CCl2)
Reaction of one molecule of HBr with one molecule of 1,3-butadiene at 400 C gives predominantly
3-bromobutene under kinetically controlled conditions
1-bromo-2-butene under thermodymically controlled conditions
3-bromobutene under thermodynamically controlled conditions
3-bromobutene under thermodynamically controlled conditions
Acid catalyzed hydration of alkenes except ethene leads to the formation of
primary alcohol
secondary or tertiary alcohol
mixture of primary and secondary alcohols
mixture of primary and secondary alcohols
