 Multiple Choice Questions
Multiple Choice QuestionsSelect the ether among following that yields methanol as one the products on reaction with cold hydroiodic acid
1-methoxybutane
1-methoxybutane-2-methylpropane
2-methoxy-2-methylpropane
methoxybenzene
Arrange the following compounds in order of decreasing acidity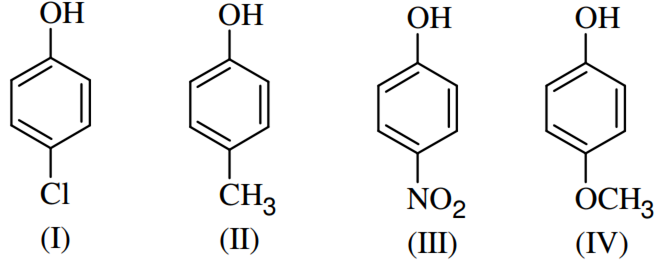
II>IV>I>III
I>II>III>IV
III>I>II>IV
III>I>II>IV
A solution of (–) –1 – chloro –1 – phenylethane is toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of
carbanion
Carbene
Carbocation
Carbocation
Iodoform can be prepared from all except
ethyl methyl ketone
isopropyl alcohol
3-methyl-2-butanone
3-methyl-2-butanone
Phenol is heated with a solution of a mixture of KBr and KBrO3.The major product obtained in the above reaction is
2-Bromophenol
3-Bromophenol
4-Bromophenol
4-Bromophenol
Which of the following reagents may be used to distinguish between phenol and benzoic acid ?
Aqueous NaOH
Tollen's reagent
Molisch reagent
Molisch reagent
From amongst the following alcohols the one that would react fastest with conc. HCl and anhydrous ZnCl2, is
1-Butanol
2-Butanol
2-Methylpropan-2-ol
2-Methylpropan-2-ol
The major product obtained on the interaction of phenol with sodium hydroxide and carbon dioxide is
Benzoic acid
Salicylaldehyde
Salicylic acid
Phthalic acid
Which of the following on heating with aqueous KOH, produces acetaldehyde?
CH3COCl
CH3CH2Cl
CH2Cl CH2Cl
CH2Cl CH2Cl
