 Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of the compound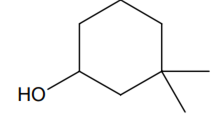
3, 3- dimethyl -1- hydroxy cyclohexane
1,1 – dimethyl -3- cyclohexanol
3,3- dimethyl -1- cyclohexanol
3,3- dimethyl -1- cyclohexanol
On mixing ethyl acetate with aqueous sodium chloride, the composition of the resultant solution is
CH3COOC2H5 + NaCl
CH3Cl + C2H5COONa
CH3COCl + C2H5OH + NaOH
CH3COCl + C2H5OH + NaOH
Acetyl bromide reacts with excess of CH3MgI followed by treatment with a saturated solution of NH4Cl given
acetone
acetyl iodide
2- methyl -2- propanol
2- methyl -2- propanol
Which one of the following reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon?
Ethyl acetate
Butan -2-one
Acetamide
Acetamide
Which of the following undergoes reaction with 50% sodium hydroxide solution to give the corresponding alcohol and acid?
Phenol
Benzoic acid
Butanal
Butanal
Phenol reacts with methyl chloroformate in the presence of NaOH to form product A. A reacts with Br2 to form product B. A and B are respectively:
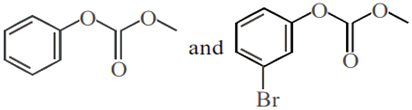
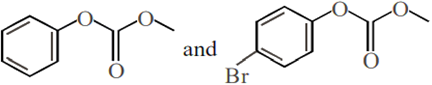
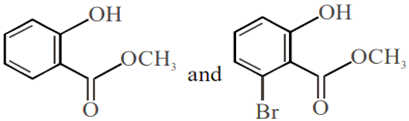
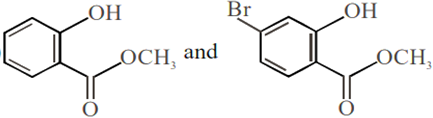
Phenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces:
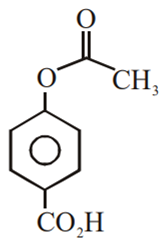
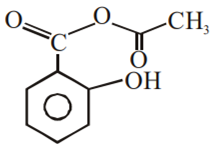
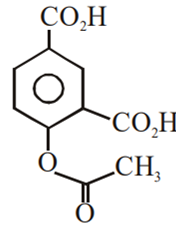
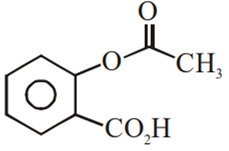
Which of the following compounds would not react with Lucas reagent at room temperature?
C6H5CH2OH
CH3CH2CH2OH
(CH3)3COH
