 Multiple Choice Questions
Multiple Choice QuestionsAs an electron makes a transition from an excited state to the ground state of a hydrogen – like atom/ion:
kinetic energy, potential energy and total energy decrease
kinetic energy decreases, potential energy increases but total energy remains same
kinetic energy and total energy decrease but potential energy increases
kinetic energy and total energy decrease but potential energy increases
The radiation corresponding to 3 → 2 transition of hydrogen atom falls on a metal surface to produce photoelectrons. These electrons are made to enter a magnetic field of 3 x 10-4 T. If the radius of the largest the circular path followed by these electrons is 10.0 mm, the work function of the metal is close to:
1.8 eV
1.1 eV
0.8 eV
0.8 eV
Hydrogen (1H1), deuterium (1H2), singly ionised helium (2He4+) and doubly ionised lithium (3Li8)2+ all have one electron around the nucleus. Consider an electron transition from n =2 to n=1. If the wavelengths of emitted radiation are λ1,λ2,λ3 andλ4, respectively for four elements, then approximately which one of the following is correct?
4λ1=2λ2=2λ3 =λ4
λ1=2λ2=2λ3 =λ4
λ1=λ2=4λ3 =9λ4
λ1=λ2=4λ3 =9λ4
In a hydrogen like atom, electron makes the transition from an energy level with quantum number n to another with a quantum number (n – 1). If n >> 1, the frequency of radiation emitted is proportional to
1/n
1/n2
1/n3/2
1/n3/2
The hydrogen atom is excited from ground state to another state with the principal quantum number equal to 4. Then the number of spectral lines in the emission spectra will be
2
3
3
3
Energy required for the electron excitation in Li++ from the first to the third Bohr orbit is
36.3 eV
108.8 eV
122.4 eV
122.4 eV
If a source of power 4kW produces 1020 photons/second, the radiation belongs to a part of the spectrum called
X -rays
ultraviolet rays
microwaves
microwaves
The potential energy function for the force between two atoms in a diatomic molecule is approximately given by 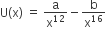 where a and b are constants and x is the distance between the atoms. If the dissociation energy of the molecule is D = [U(x = ∞) – Uat equilibrium], D is
where a and b are constants and x is the distance between the atoms. If the dissociation energy of the molecule is D = [U(x = ∞) – Uat equilibrium], D is
b2/2a
b2/12a
b2/4a
b2/4a
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths r = λ1/λ2, is given by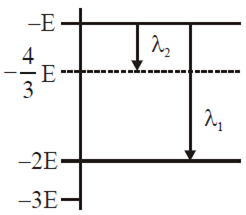
r = 3/4
r = 1/3
r = 4/3
r = 4/3
Suppose an electron is attracted towards the origin by a force k/r where ‘k’ is a constant and ‘r’ is the distance of the electron from the origin. By applying Bohr model to this system, the radius of the nth orbital of the electron is found to be ‘rn’ and the kinetic energy of the electron to be Tn. Then which of the following is true?
Tn ∝ 1/n2, rn ∝ n2
Tn independent of n, rn ∝ n
Tn ∝ 1/n, rn ∝ n
Tn ∝ 1/n, rn ∝ n
