 Multiple Choice Questions
Multiple Choice QuestionsConsider the following reactions: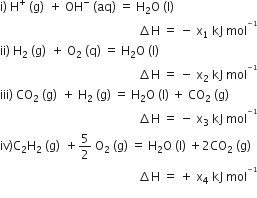
Enthalpy of formation of H2O (l) is:
- x2 kJ mol-1
+ x3 kJ mol-1
- x4 kJ mol-1
- x4 kJ mol-1
Given that bond energy of H-H and Cl- Cl are 430 kJ mol-1 and 240 kJ mol-1 respectively and ΔHf for HCl is -90 kJ mol-1. Bond and ΔHf for HCl is -90 kJ mol-1.Bond enthalpy of HCl is:
290 kJ mol-1
380 kJ mol-1
425 kJ mol-1
425 kJ mol-1
Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔE?
H2 (g) + Br2 (g) →2HBr (g)
C (s) + 2 H2O (g) → 2 H2 (g) + CO2 (g)
PCl5 (g) →PCl3 (g) + Cl2 (g)
PCl5 (g) →PCl3 (g) + Cl2 (g)
The enthalpy of combustion of H2, cyclohexene (C6H10) and cyclohexene (C6H12) are -241, -3800 and -3920 kJ per mol respectively.The heat of hydrogenation of cyclohexane is:
-212 kJ mol
+121 kJ mol
+242 kJ per mol
+242 kJ per mol
A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy ΔU of the gas in joules will be
1136.5 J
-500 J
-505 J
-505 J
For a given reaction, ΔH = 35.5 kJ mol–1 and ΔS = 83.6 JK–1 mol–1. The reaction is spontaneous at (Assume that ΔH and ΔS do not vary with temperature)
T < 425 K
T > 425 K
All temperatures
All temperatures
Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below :
Then the species undergoing disproportionation is
HBrO
Br2
Which one of the following conditions will favour maximum formation of the product in the reaction,
Low temperature and high pressure
Low temperature and low pressure
High temperature and low pressure
High temperature and high pressure
The bond dissociation energies of X2, Y2 and XY are in the ratio of 1 : 0.5: 1. ΔH for the formation of XY is –200 kJ mol–1. The bond dissociation energy of X2 will be
200 kJ mol-1
100 kJ mol-1
400 kJ mol-1
800 kJ mol-1
The correction factor ‘a’ to the ideal gas equation corresponds to
Density of the gas molecules
Volume of the gas molecules
Forces of attraction between the gas molecules
Electric field present between the gas molecules
