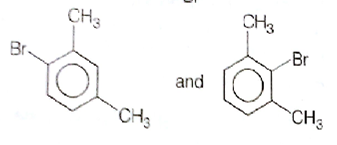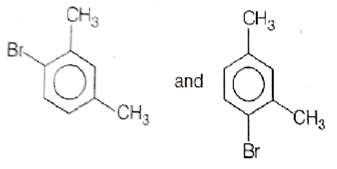Multiple Choice Questions
Multiple Choice Questions2-Phenylethylbromide when heated with NaOEt, elimination takes place. No deuterium exchange takes place when the reaction is carried out in C2H5OD solvent. The mechanism will be
E1 elimination
E2 elimination
E1 cB elimination
E2 or E1cB
For the following reactions,
i) CH3 CH2 CH2Br + KOH --> CH3CH=CH2 +KBr +H2O
ii) 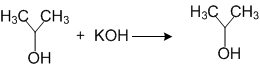
iii) 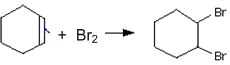
(i) is elimination reaction, (ii) is substitution reaction, and (iii) is addition reaction.
(i) is elimination reaction, (ii) is addition reaction, (iii) is substitution reactions.
(i) is substitution, (ii) addition reaction (iii) is addition reactions
(i) is substitution, (ii) addition reaction (iii) is addition reactions
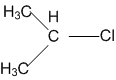
In which of the following compounds, the C-Cl bond ionisation shall give most stable carbonium ions?
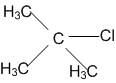
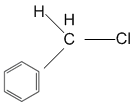
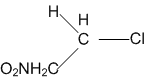
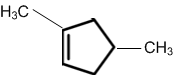
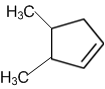
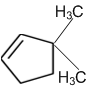
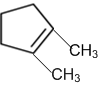
A single compound of the structure is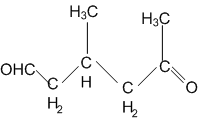
obtainable from ozonolysis of which of the following cyclic compound?
Which of the following is a correct electron displacement for a nucleophilic reaction to take place?
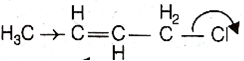

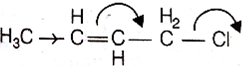
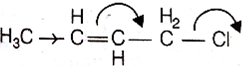
Given,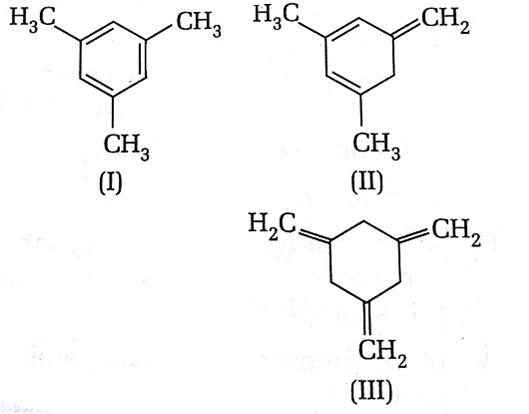
The enthalpy of hydrogenation of these compounds will be in the order as
I>II>III
I<II<III
II>III>I
II>III>I
Identity Z in the sequence of reactions.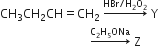
CH3-(CH2)3-O-CH2CH3
(CH3)2CH2-O-CH2CH3
CH3(CH2)4-O-CH2CH3
CH3(CH2)4-O-CH2CH3
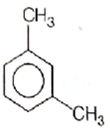
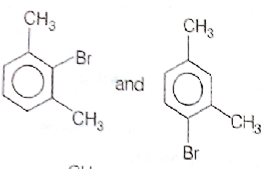
What products are formed when the following compounds is treated with Br2 in the presence of FeBr3?