 Long Answer Type
Long Answer Type(a) Complete the following chemical equation
(i) Cu + HNO3 (dilute) --->
(ii) XeF4 + O2F2 -->
(b) Explain the following observation:
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) Oxygen is a gas but sulphur a solid.
(iii) The halogens are coloured. Why?
a) Define the following terms:
(i) Mole fraction
(ii) Ideal solution
(b) 15.0 g of an unknown molecular material is dissolved in 450 g of water. The resulting solution freezes at - 0.34°C. What is the molar mass of the material? (Kf for water = 1.86 K kg mol-1)
(a) Explain the following:
(i) Henry’s law about the dissolution of a gas in a liquid.
(ii) Boiling point elevation constant for a solvent.
(b) A solution of glycerol (C3H8O3) in water was prepared by dissolving some glycerol in 500 g of water. This solution has a boiling point of 100.42°C. What mass if glycerol was dissolved to make this solution? (Kb for water = 0.512 K kg mol-1)
(a) Write a suitable chemical equation to complete each of the following transformations:
(i) Butan-1-ol to butanoic acid
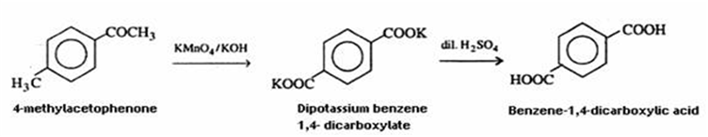
(ii) 4-methylacetophenone to benzene-1, 4-dicarboxylic acid
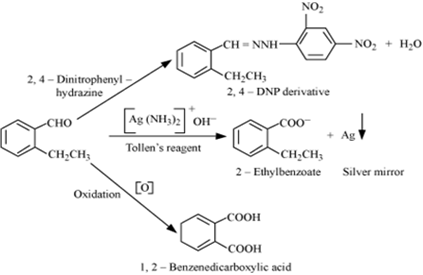
(b) An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro’s reaction. On vigorous oxidation, it gives 1, 2-benzenedicarboxylic acid. Identify the compound.
(i) Butanoic acid can be obtained by oxidation of butan-1-ol.
The most common reagent used for oxidation of alcohols is chromium (Vl) reagents including chromic acid (H2CrO4), potassium dichromate (K2Cr2O7) and chromic anhydride (CrO3).
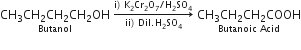
(ii) 4- methylacetophenone can be converted to benzene-1, 4- dicarboxylic acid as follows:
(b) It is given that the compound (with molecular formula C9H10O) forms 2, 4-DNP derivative and reduces Tollen’s reagent. Therefore, the given compound must be an aldehyde.
Again, the compound undergoes Cannizzaro reaction and on oxidation gives 1, 2-benzenedicarboxylic acid. Therefore, the -CHO group is directly attached to a benzene ring and this benzaldehyde is ortho-substituted. Hence, the compound is 2-ethylbenzaldehyde.
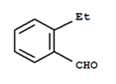
2 – ethyl bezaldehyde
The given reactions can be explained by the following equations.
(a) Give chemical tests to distinguish between
(i) Propanol and propanone
(ii) Benzaldehyde and acetophenone
(b) Arrange the following compounds in an increasing order of their property as indicated:
(i) Acetaldeyde, Acetone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) Benzoic acid, 3, 4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength).
(iii) CH3CH2CH (Br) COOH, CH3CH (Br) CH2COOH, (CH3)2CHCOOH (acid strength)
