 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following, not a correct statement?
The electron -deficient molecules can act as Lewis acids
The canonical structures have no real existence
Every AB5 molecules does, in fact, have square pyramid structure
Every AB5 molecules does, in fact, have square pyramid structure
C.
Every AB5 molecules does, in fact, have square pyramid structure
Every AB5 molecule does not, in fact, have square pyramidal structure but AB5 molecules have trigonal bipyramidal structures due to sp3d hybridisation.
The general molecular formula, which represents the homologous series of alkanols is:
CnH2nO2
CnH2nO
CnH2n+1O
CnH2n+1O
The correct order of the mobility of the alkali metal ions in aqueous solution is:
Li+>Na+ > K+ > Rb+
Na+>K+ > Rb+ > Li+
K+ > Rb+ >Na+ > Li+
K+ > Rb+ >Na+ > Li+
The correct regarding the electronegativity of hybrid orbitals of carbon is:
sp> sp2<sp3
sp> sp2 > sp3
sp< sp2 > sp3
sp< sp2 > sp3
The IUPAC name of is: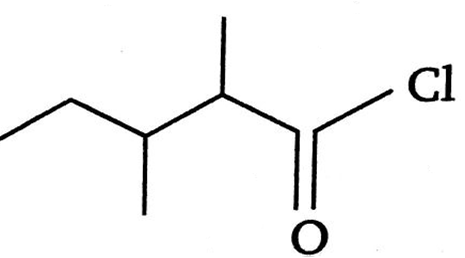
3,4-dimethylpentanoyl chloride
1-chloro-1-oxo-2, 3-dimethyl pentane
2-ethyl -3-ethylbutanonyl chloride
2-ethyl -3-ethylbutanonyl chloride
A solution containing 10 g per dm3 is urea (molecular mass = 60 g mol-1) is isotonic with a molecular mass of this non-volatile solute. The molecular mass of this of this non-volatile solute is:
250 g mol-1
300 g mol-1
350 g mol-1
350 g mol-1
A plot of log x/m versus log p for the adsorption of a gas on a solid gives a straight line with slope equal to:
-log k
n
1/n
1/n
CsBr crystallises in a body centred cubic lattice. The unit cell length is 436.6 pm. Given that the atomic mass of Cs - 133 and that of Br = 80 amu and Avogadro number being
6.02 x 1023 mol-1, the density of CsBr is:
42.5 g/cm3
0.425 g/cm3
8.25 g/cm3
8.25 g/cm3
More the number of oxidation states are exhibited by the actinoids than by the lanthanoids. The main reason for this is:
more energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals
the lesser energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals
the greater metallic character of the lanthanoids than that of the corresponding actinoids
the greater metallic character of the lanthanoids than that of the corresponding actinoids
