 Multiple Choice Questions
Multiple Choice QuestionsThe reagent in Friedel-Craft's reaction is :
pyridine
RCOCl
RCOOH
HCl
B.
RCOCl
The reaction alkyl halide or acyl halide react with benzene in the presence of anhydrous AICl3 to form homologues of benzene is known as Friedel-Craft's reaction .
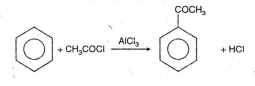
In which of the following reactions carbon-carbon bond formation takes place ?
Cannizaro
Reimer - Tiemann
HVZ reaction
Schmidt reaction
Which gives only mono - substituted product ?
o - dinitrobenzene
m - dinitrobenzene
p - dinitrobenzene
Nitrobenzene
Order of boiling point is :
HF > HI > HBr > HCl
HF > HBr > HI > HCl
HCl > HBr > HI > HF
HCl > HI > HBr > HF
If the enolate ion combines with carbonyl group of ester , we get :
aldol
, - unsaturated ester
- keto aldehyde
acid
Which of the following compounds will react with NaHCO3 solution to give sodium salt and carbon dioxide ?
Acetic acid
n - hexanol
Phenol
Both (b) and (c)
