 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following reactions will not give propane ?
CH3CH2CH2Cl
CH3COCl
CH3CH=CH2
CH3-C(OH)-CH3
A compound A ➔ C5H10Cl2 on hydrolysis gives C5H10O which reacts with NH2OH forms iodoform , but does not give Fehling test A is :
![]()
![]()
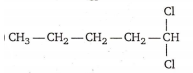
![]()
A.
![]()
A compound (C5H10Cl2) on hydrolysis gives C5H10O , which reacts with hydroxylamine , so C5H10O must be aldehydic or ketonic group but it does not give Fehling test , so it must be a ketone and it form iodoform which is a charactertistic test of CH3CO group .
In photography , sodium thiosulphate is used as :
complexing agent
oxidising agent
reducing agent
none of these
Which of the following is not a broad spectrum antibiotic ?
Tetracycline
Chloromycetin
Penicillin
None of these
The IUPAC name of compound is :
CH2=CH-CH(CH3)2
1 - isopropylethylene
2 - vinyl propane
3 - methyl 1 - butene
1 , 1 , dimethyl 1 , 2 - propene
Which of the following test is not used for testing of proteins ?
Millon's test
Molisch's test
Biuret test
Ninhydrin test
