 Multiple Choice Questions
Multiple Choice QuestionsIn the hydrocarbon
The state of hybridization of carbon 1, 3, and 5 are in the following sequence
sp2, sp, sp3
sp, sp3, sp2
sp, sp2, sp3
sp, sp2, sp3
The value of KP1 and Kp2 for the reactions
X ⇌ Y +Z ... (i)
A ⇌ 2B ... (ii)
are in the ratio of 9:1. if the degree of dissociation of X and A be equal, then the total pressure at equilibrium (i) and (ii) are in the ratio
3:1
1:9
36:1
36:1
The value of equilibrium constant of the reaction HI (g) ⇌ H2(g)/2 + I2/2 is 8.0
The equilibrium constant of the reaction H2(g) + I2(g) ⇌ 2 HI (g) will be
1/16
1/64
16
16
Green chemistry means such reactions which
produce colour during reactions
reduce the use and production of hazardous chemicals
are related to the depletion of the ozone layer
are related to the depletion of the ozone layer
The alkali metals form salt-like hydrides by the direct synthesis at elevated temperature. The thermal stability of these hydrieds decreases in which of the following orders?
CsH > RbH > KH > NaH >LiH
KH > NaH> LiH > CsH >RbH
NaH > LiH >KH> RbH> CsH
NaH > LiH >KH> RbH> CsH
The stability of carbanions in the following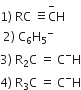
is in the order of
(1) > (2) > (3) > (4)
(2) > (3) > (4) > (1)
(4) > (2) > (3) > (1)
(4) > (2) > (3) > (1)
On the basis of the following Eo values; the strongest oxidising agent is
[Fe(CN)6]4- → [Fe(CN)6]3-] +e- ;
Eo = -0.35 V
Fe2+ → Fe3+ +e-; E = -0.77 V
[Fe(CN)6]4-
Fe2+
Fe3+
Fe3+
Kohlrausch's law states that at
finite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.
infinite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte depending on the nature of the other ion of the electrolyte.
infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever be the nature of the other ions of the electrolyte.
infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever be the nature of the other ions of the electrolyte.
Volume occipied by one molecule of water (density = 1 g cm-3) is
9.0 x 10-23
6.023 x 10-23 cm3
3.0 x 10-23 cm3
3.0 x 10-23 cm3
Which of the following complexes exhibits the highest paramagnetic behaviour?
Where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities
(At. no. ; Ti = 22, V = 23, Fe =26, Co = 27)
[V(gly)2 (OH)2 (NH3)2]+
[Fe(en)(bpy)(NH3)2]+
[CO(OX)2(OH)2]-
[CO(OX)2(OH)2]-
C.
[CO(OX)2(OH)2]-
Greater is the number of unpaired electrons, larger is the paramagnetism.
[V(gly)2(OH)2(NH3)2]+
V23 = [Ar] 4s2, 3d2
Oxidation state of V in [[V(gly)2(OH)2(NH3)2]+ is
x + (-1) x 2 + (-1) x 2 + (0) x 2 = +1
x=+5
V5+ = [Ar]3d0
[Fe(en)(bby)(NH3)2]2+
x + (0) + (0) + (0) x 2 = +2
x = +2
