 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following is an amine hormone?
Thyroxine
Oxypurin
Insulin
Progesterone
A.
Thyroxine
The hormone which is proteinous nature are said to be amine hormone because protein is the polymers of polypeptides and polypeptides are made up of amino acids. An amino acid contains amino group.
Thyroxin is an amine hormone. It is secreted by the thyroid gland. Its function is to control the metabolism of carbohydrates, proteins and lipids.
Which one of the following statements is not true?
In vulcanization, the formation of sulphur bridges between different chains make rubber harder and stronger
Natural rubber has the trans-configuration at every double bond
Buna-S is a copolymer of butadiene and styrene
Natural rubber is a 1,4 polymer of isoprene
In DNA. the complimentary bases are
Adenine and thymine; guanine and cytosine
Adenine and thymine; guanine and uracil
Adenine and guanine, thymine: Guanine and uracil
Uracil and adenine; cytosine and guanine
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure
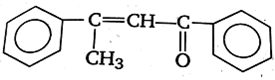
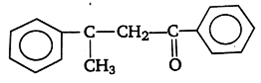
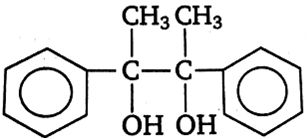
![]()
The relative reactivities of acyl compounds towards of nucleophilic substitution are in the order of
Acyl chloride> Acid anhydride> Ester> Amide
Ester > Acyl chloride> Amide > Acid anhydride
Acid anhydride > Amide > Ester> Acyl chloride
Acyl chloride > Ester > Acid anhydride > Amide
In an SN2 substitution reaction of the type which ![]() one of the followings has the highest relative rate?
one of the followings has the highest relative rate?
CH3-CH2-CH2Br
CH3-CH(CH2)-CH2Br
CH3C(CH3)2CH2Br
CH3CH2Br
