 Multiple Choice Questions
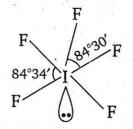
Multiple Choice QuestionsThe structure of IF5 can be best described as :
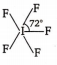
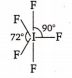
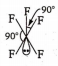
None of these
D.
None of these
Number of hybrid orbitals = number of bp + number of lp
= 5 + 1 = 6
Thus , hybridisation is sp3d2 but geometry , due to the presence of one lone pair , is square pyramidal , i.e. ,
From elementary molecular orbital theory we can deduce the electronic configuration of the singly positive nitrogen molecular ion as :
1s2 , 1s2 , 2s2 , 2s2 , 2p4 , 2p1
1s2 , 1s2 , 2s2 , 2s2 , 2p2 , 2p3
1s2 , 1s2 , 2s2 , 2s2 , 2p3 , 2p2
1s2 , 1s2 , 2s2 , 2s2 , 2p2 , 2p4
A solution contains 25% H2O , 25% C2H5OH and 50% CH3COOH by mass .The mole fraction of H2O would be :
0.25
2.5
0.502
5.03
If the solubility of PbCl2 at 25°C is 6.3 x 10-2 mol/L , its solubility product is :
1 x 10-6
1 x 10-3
1.1 x 10-6
1.1 x 10-5
A double bond connecting two atoms .There is a sharing of :
2 electrons
1 electron
4 electrons
All electrons
