 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following carbonyl will have the strongest C- O bond?
(Mn(CO)6+
Cr(CO)6
V (CO)6-
V (CO)6-
A.
(Mn(CO)6+
As the positive charge on the central metal atom increase, the less readily the metal can donate electron density into the anti - bonding pi-orbitals of CO ligand to weaken the C-O bond. Hence, the C-O bond would be strongest in Mn(CO)6+.
The rate of reaction 2N2O3 → 4 NO2 + O2 can be written in three ways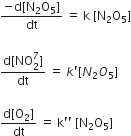
The relationship between k and k' and between k and k'' are
k' = 2k ; k' = k
k' = 2k; k" = k/2
k' = 2k; k' = 2k
k' = 2k; k' = 2k
A solid compound XY has NaCl structure. If the radius of the cation is 100 pm, the radius of the anion (Y-) will be
275.1 pm
322.5 pm
241.5 pm
241.5 pm
A solution contains Fe2+ Fe3+, and I- ions. This solution was treated with iodine at 35o C.Eo for Fe3+/ Fe2+ is +0.77 V and Eo for I2/ 2 I- = 0.536 V. The favourable redox reaction is
I2 will be reduced to I-
There will be No redox reaction
I- will be oxidised to I2
I- will be oxidised to I2
The unit of rate constant for a zero order reaction is
mol L-1 s-1
L mol-1 s-1
L2 mol-2 s-1
L2 mol-2 s-1
200 mL of an aqueous solution of a protein contains its 1.26 g . The osmotic pressure of this solution at 300 K is found to be 2.57 x 10-3 bar. The molar mass of protein will be (R = 0.083 L bar mol-1 K-1)
51022 g mol-1
122044 g mol-1
31011 g mol-1
31011 g mol-1
In qualitative analysis, the metals of group I can be separted from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and Pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl- concentration is 0.10 M. What will be the concentration of Ag+ and Pb2+ be at equilibrium? (Ksp for AgCl = 1.8 x 10-10, Ksp for PbCl2 = 1.7 x 10-5)
[Ag+] = 1.8 x 10-7 M; [Pb2+] = 1.7 x 10-6 M
[Ag+] = 1.8 x 10-11 M; [Pb2+] = 8.5 x 10-5 M
[Ag+] = 1.8 x 10-9 M; [Pb2+] = 1.7 x 10-3 M
[Ag+] = 1.8 x 10-9 M; [Pb2+] = 1.7 x 10-3 M
The pair of species of oxygen and their magnetic behaviours are noted below. Which of the following presents the correct description?




The following reactions take place in the blast furnace in the preparation of impure iron. Identify the reaction pertaining to the formation of the slag.
Fe2O3 (s) + 3CO (g) → 2 Fe (l) +3CO2 (g)
CaCO3 (s) → CaO (s) + CO2 (g)
CaO (s) +SiO2 → CaSiO3 (s)
CaO (s) +SiO2 → CaSiO3 (s)
