 Multiple Choice Questions
Multiple Choice Questionshave
S-S bond
S-O bridge
O-O bridge
All S-O bond lengths are same.
C.
O-O bridge
The stmcture of peroxodisulfate anion is:
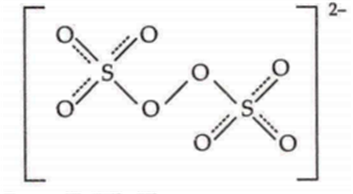
It has a O-O bridge.
Assertion : Diamond and graphite do not have the same crystal structure.
Reason : Diamond is crystalline while graphite is amorphous.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Bohr model fails in case of multielectron pecies.
Reason : It does not mention electron-electron interactions.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : ClF3 has T-shape structure.
Reason : It has two lone pairs arranged at 180° angle.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false
Assertion : O2 is paramagnetic.
Reason : It has one unpaired electron.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : In a pressure cooker, the water is brought to boil. The cooker is then removed from the stove. Now on removing the lid of pressure cooker, the water starts boiling again.
Reason: The impurities in water bring down its boiling point.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
For silicon which is not correct?
It is a type of silicate.
It is thermally unstable.
It is hydrophilic.
Repeating unit is R2SiO.
If Si is doped with B,
n-type semiconductor is formed
p-type semiconductor is formed
insulator is formed
polymer is formed.
Living in the atmosphere of CO is dangerous, because it
combines with O, present inside to form CO2
reduces organic matter of tissues
combines with haemoglobin and makes it incapable to absorb oxygen
dries up the blood.
