Multiple Choice Questions
Multiple Choice QuestionsWhich of the following statements about the interstitial compounds is incorrect?
They retain metallic conductivity
They are chemically reactive
They are much harder than the pure metal
They have higher melting points than the pure metal
6.02 x 1020 molecules of urea are present in 100 mL of its solution. The concentration of solution is
0.02 M
0.01 M
0.001 M
0.1 M
KMnO4 can be prepared from K2MnO4 as per reaction
The reaction can go to completion by removing OH- ions by adding
HCl
KOH
CO2
SO2
Identify the correct order of solubility in aqueous medium.
CuS > ZnS > Na2S
ZnS > Na2S > CuS
Na2S > CuS > ZnS
Na2S > ZnS > CuS
Which of these is not a monomer for a high molecular mass silicone polymer?
MeSiCl3
Me2SiCl2
Me3SiCl
PhSiCl3
C.
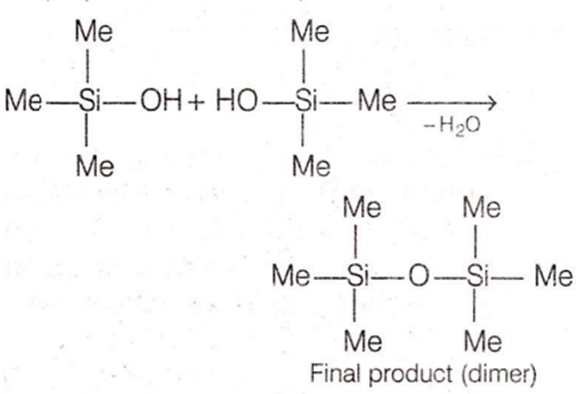
Me3SiCl
Me3SiCl is not a monomer for a high molecular mass silicone polymer because it generates Me3SiOH when subjected to hydrolysis which contains only one reacting site. Hence, the. polymerisation reaction stops just after first step.