 Multiple Choice Questions
Multiple Choice QuestionsMY and NY3, two nearly insoluble salts. have the same Ksp values of 6.2 x 10-13 at room temperature. Which statement would be true in regard to MY and NY3 ?
The molar solubility of MY in water is less than that of NY3
The salts MY and NY3 are more soluble in 0.5 M KY than in pure water
The addition of the Salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
The addition of the Salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
A.
The molar solubility of MY in water is less than that of NY3
For MY,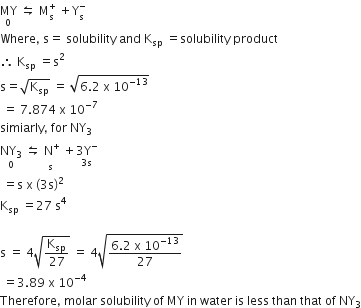
Consider the following liquid-vapour equilibrium
Which of the following relations is correct?
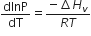

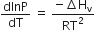
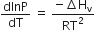
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is
The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain
The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain
The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain
The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain
Which of the following statement about hydrogen is incorrect?
Hydrogen never acts as cation in ionic salts
Hydronium ion, H3O+ exist freely in solution
Dihydrogen does not act as a reducing agent
Dihydrogen does not act as a reducing agent
When copper is heated with conc. HNO3 it produces
Cu(NO3)2 and NO
Cu(NO3)2, NO and NO2
Cu(NO3)2 and N2O
Cu(NO3)2 and N2O
A 1000C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732 mm. If Kb = 0.52, the boiling point of this solution will be
1000C
1020C
1030C
1030C
Match the compounds given in column I with the hybridization and shape given in column II and mark the correct option.
|
Column I |
Column II |
|
A.XeF6 |
1.Distorted octahedral |
|
B.XeO3 |
2.Square planar |
|
C.XeOF4 |
3.Pyramidal |
|
D.XeF4 |
4.Square pyramidal |
|
A
|
B
|
C
|
D
|
|
A
|
B
|
C
|
D
|
|
A
|
B
|
C
|
D
|
|
A
|
B
|
C
|
D
|
Match items of Columns I with the items of Columns II and assign the correct code.
|
Column I |
Column II |
|
A.Cyanide process |
1.Ultrapure Ge |
|
B. Froth floatation process |
2.Dressing of ZnS |
|
C.Electrolytic reduction |
3.Extraction of Al |
|
D.Zone refining |
4.Extraction of Au |
|
|
5.Purification of Ni |
|
A
|
B
|
C
|
D
|
|
A
|
B
|
C
|
D
|
|
A
|
B
|
C
|
D
|
|
A
|
B
|
C
|
D
|
Which is the correct statement for the given acids?
Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid.
phosphinic acid is a diprotic acid while phosphonic acid is a monoprotic acid
Both are triprotic acids
Both are triprotic acids
Which one of the following statements is correct when SO2 is passed through acidified K2Cr2O7 solution?
The solution is decolourized
SO2 is reduced
Green Cr2(SO4)3 is formed
The solution turns blue
