 Multiple Choice Questions
Multiple Choice Questions100 mL of a solution contains 2 g of acetic acid and 3g of sodium acetate providing Ka =1.8 x 10-5 , then choose the correct option.
This solution is basic in nature
This solution is acidic in nature
This solution is amphoteric in nature
This solution is neutral in nature
Consider the following structures
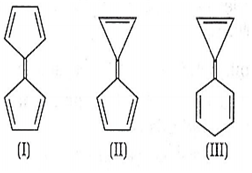
Choose the correct statement regarding the above structures.
Dipole moment varies as II > III > I
II is more stable than I
I is the most reactive among three
All of the above
Both Mg and Fe metal can reduce copper from a solution having Cu2+ ion, according to equilibria.
Mg(s) + Cu2+ Mg2+ + Cu(s); K1 = 6 x 1090
Fe(s) + Cu2+ Fe2+ + Cu(s); K2 = 3 x 1026
Choose the correct option regarding above equilibrium
Mg removes more Cu2+ from solution
Fe removes more Cu2+ from solution
Both will equally remove Cu2+ from solution
Both metals cannot remove Cu2+ from solution
Match the particle with its characteristic
| Column I | Column II |
| A. -particle | p. Slow moving |
| B. Isobar | q. High penetration power |
| C. - ray | r. Same atomic mass |
| D. - particle | s. consists of electron |
A - p; B - r; C - q; D - s
A - p; B - q; C - r; D - s
A - r; B - s; C - p; D - q
A - s; B - r; C - p; D - q
Highest energy will be absorbed to eject out the electron in the configuration.
1s22s22p1
1s22s22p3
1s22s22p2
1s22s22p4
B.
1s22s22p3
Highest electric will be absorbed to eject out the electron in the configuration of 1s22s22p3.
At radioactive equilibrium, the ratio between two atoms of radioactive elements A and B is 3.1 x 109 : 1. If the half-life period of A is 2 x 1010 yrs, then the half-life of B is
9.54 yrs
2.14 yrs
3.29 yrs
6.45 yrs
When 2-methyl butyl bromide is treated with sodium ethoxide in ethanol, what will be the major product?
2-methyl but-2-ene
3-methyl but-1-ene
2-methyl but-1-ene
2-methyl sodium-butoxide
Which of the following hydrocarbons is the most reactive towards addition of H2SO4?
Ethene
Propylene
3-methyl but-1-ene
1-butene
For the formation of Cr2O3 and Al2O3, values of are 540 kJ mol-1 and -827 kJ mol-1 respectively. What will be the possibility for reaction of Cr2O3 by Al?
Reduction of Cr2O3 by Al will take place
Oxidation of Cr2O3 by Al will take place
Neither oxidation nor reduction will take place
Reaction is not feasible
