 Multiple Choice Questions
Multiple Choice QuestionsAssertion : Generally alkali and alkaline earth metals form superoxides.
Reason: There is a single bond between O and O in superoxides.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false
D.
If both assertion and reason are false
Only K, Rb and Cs from alkali metals form superoxides and superoxides possess three electron bond (: :)-.
Assertion : For hydrogen like species, energy of an electron in a particular orbit increases with increase in value of Z.
Reason : Electronegativity decreases across a period.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false
Assertion : C- - H bond angle is less than the normal tetrahedral bond angle.
Reason : Lone pair-lone pair repulsion decreases bond angle.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Critical temperature of CO2 is 304 K, it cannot be liquified above 304 K.
Reason : At a certain temperature, volume
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Diamagnetic substances are not attracted by magnetic field.
Reason : Diamagnetic substances have no unpaired electrons.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false
Assertion : A reaction which is spontaneous and accompanied by decrease of randomness must be exothermic.
Reason : All exothermic reactions are accompanied by decrease of randomness.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
Assertion : H2S is stronger acid than PH3.
Reason : S is more electronegative than P, conjugate base HS- is more stable than H2P-.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Heat of neutralisation for both H2SO4 and HCl with NaOH is 53.7 kJ mol-1.
Reason : Both HCl and H2SO4 are strong acids.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false.
For which of the following elements it is difficult to disproportionate in +3 oxidation state ?
N
As
Sb
Bi
Ease of nucleophilic addition in the given compounds is
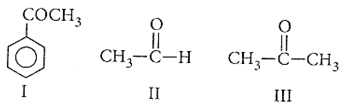
I > III > II
II > III > I
II > I > III
III > I > II
