
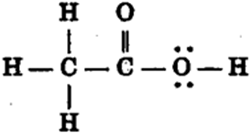
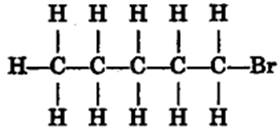
Draw the structures for the following compounds.
(i) Ethanoic acid (ii) Bromopentane*
(iii) Butanone (iv) Hexanal.
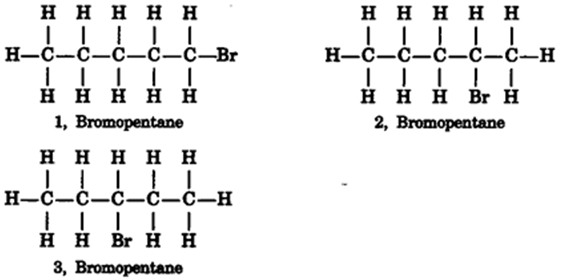
Are structural isomers possible for bromopentane?
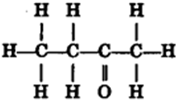

When ethyne is burnt in air, it gives a sooty flame. This is due to incomplete combustion caused by limited supply of air. However, if ethyne is burnt with oxygen, it gives a clean flame with temperature 30000 C because of complete combustion. This oxy-acetylene flame is used for welding. It is not possible to attain such a high temperature without mixing oxygen. That is why a mixture of ethyne and air is not used.![]()
Conversion of ethanol to ethanoic acid is oxidation because
(i)Oxygen is added up to ethanol by oxidising agent like alkaline potassium permanganate or acidified potassium dichromate.
(ii)The product of the reaction is not CO2 and water. Further no heat or light is produced.
C2H5OH CH3COOH
