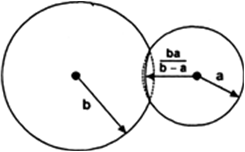
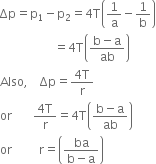
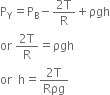
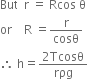
Statement When a body is immersed partially or wholly in a liquid, its weight appears to be reduced and loss of weight is equal to the weight of liquid displaced by the body.
Proof: Consider a body of height h and area of cross-section A placed in liquid of density σ at a depth x below the free surface. Let ρ be density of the body.
Now the pressure at the upper face of the bodi is,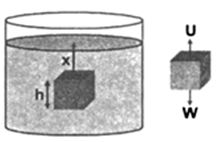
Atmosphere is a gaseous envelope surrounding the earth and the pressure exerted by the atmosphere is called atmospheric pressure.
The atmosphere exerts a huge pressure. The cause of atmospheric pressure is motion of air molecule. The air molecules in continuous motion strike the surface of body placed in it and exert a huge force.
To measure the atmospheric pressure, Torricelli took a meter long graduated tube and he filled it with clean and dry mercury. By closing the tube with thumb, he inverted the tube in a cistern ( a tub filled with mercury) as shown in the figure. He observed that the level of mercury first fell down and finally stayed with a column BC = 76cm in the tube above the free surface of mercury in the cistern leaving behind vacuum above C.
In the tube, above C there is vacuum, therefore pressure at point C is zero. Point B in the tube is 76cm below C, therefore pressure at B is,
PB =PC + ρ8h = ρ8h
where ρ is density of mercury and h be the height column of mercury in the tube above point B.
In the figure, point A is at the interface of air and mercury, therefore it is both in air and as well as in mercury. Thus pressure at point A is equal to atmospheric pressure, i.e.
PA = Atmospheric pressure As vertical height between A and B is zero, therefore pressure at A and B is same, i.e.
![]()
or Atmospheric pressure = pgh
= 13600 x 9.8 x 0.76
= 1.013 x 105 N/m2

Therefore there is more pressure inside than outside. Let pi be the pressure inside the liquid drop and po be the pressure outside the drop. Therefore excess of pressure inside the liquid drop is,
p = p1– p0
Due to excess of pressure inside the liquid drop the free surface of the drop will experience the net force in outward direction due to which the drop will expand. Let the free surface displace by dR under isothermal conditions. Therefore excess of pressure does the work in displacing the surface and that work will be stored in the form of potential energy.
The work done by excess of pressure in displacing the surface is,
dW = Force x displacement
= (Excess of pressure x surface area) x displacement of surface
![]()
Increase in the potential energy is,
dU = surface tension x increase in area of the free surface
![<pre>uncaught exception: <b>mkdir(): Permission denied (errno: 2) in /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/util/sys/Store.class.php at line #56mkdir(): Permission denied</b><br /><br />in file: /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/util/sys/Store.class.php line 56<br />#0 [internal function]: _hx_error_handler(2, 'mkdir(): Permis...', '/home/config_ad...', 56, Array)
#1 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/util/sys/Store.class.php(56): mkdir('/home/config_ad...', 493)
#2 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/FolderTreeStorageAndCache.class.php(110): com_wiris_util_sys_Store->mkdirs()
#3 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/RenderImpl.class.php(231): com_wiris_plugin_impl_FolderTreeStorageAndCache->codeDigest('mml=<math xmlns...')
#4 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/TextServiceImpl.class.php(59): com_wiris_plugin_impl_RenderImpl->computeDigest(NULL, Array)
#5 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/service.php(19): com_wiris_plugin_impl_TextServiceImpl->service('mathml2accessib...', Array)
#6 {main}</pre>](/application/zrc/images/qvar/PHEN11039425-2.png)
