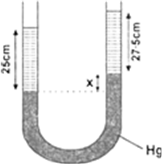
∴ Pressure due to 25 cm of water
= Pressure due to x cm of mercury
+ Pressure due to 27.5 cm of spirit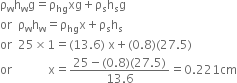
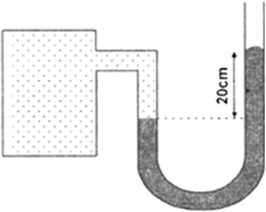
A manometer reads the pressure of a gas in an enclosure as shown in the figure. The liquid used in the manometers is mercury and the atmospheric pressure is 76 cm of mercury.
Give the absolute and gauge pressure of the gas in the enclosure.