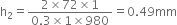
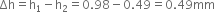
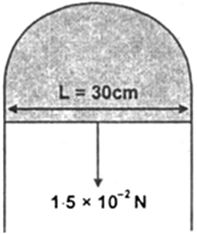
Therefore, total length of free surface that touches with slider is 2L = 60cm = 0.60 m.
Because the slider can support 1.5x10–2N weight.
If an air bubble of 5.0 mm is formed at depth of 40.0 cm inside a container containing soap solution (of relative density 1.20), what would be the pressure inside the bubble?(1AP = 1.013 x105 Pa and surface tension of soap solution is 0-025 N/m)
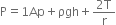
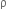 =1.2 gm/cc = 1200 kg/m3
=1.2 gm/cc = 1200 kg/m3 
Here,
Angle of contact,
Radius of the narrow tube, r = 1.0mm=10-3m
Surface tension, T = 0.465 N/m
Density of mercury, = 13600 kg/m3
We know the height by which liquid rises in the tube is,
Negative sign means that mercury will dip down.
