When we apply deforming force on a body, the shape of the body changes and body is said to be strained. Strain is measured as the ratio of change in configuration to the original configuration.
There are three type of strains: (i) Longitudinal strain: When the deforming force produces a change in length only then the strain produced in the body is called longitudinal strain. The longitudinal strain is measured as the ratio of change in length (∆L) of body to its original length L.![]()
(ii) Volumetric strain: When the deforming force produces a change in volume only then the strain produced in the body is called volumetric strain. The volumetric strain is measured as the ratio of change in volume (∆V) of the body to its original volume V.![]()
(iii) Shearing strain: When the deforming force produces a change only in shape of the body without changing its volume then the strain produced in the body is called sheer strain. It is the ratio of the relative displacement of one plane to its perpendicular distance from the fixed plane.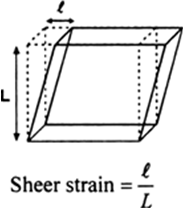
All types of strains are dimensionless.

