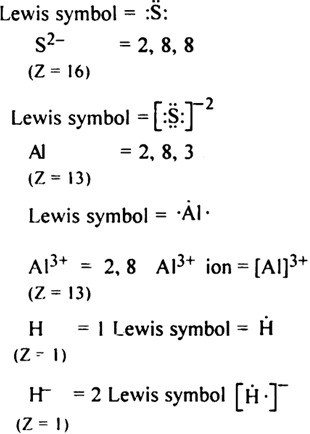Draw the Lewis structures for the following molecules and ions:![]()
Lewis structure of the given molecule and ions are,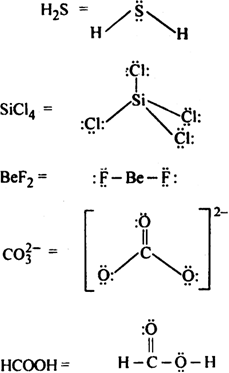
Lewis dot structure of given elements are: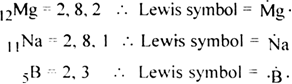
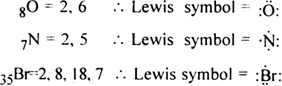
Explain the formation of a chemical bond.
The attractive force which holds various constituents (atoms,ions, etc.)together in different chemical species is called a chemical bond.
Molecules having two identical atoms like H2 and O2 Cl2 N2 are called as homonuclear diatomic molecules.
Molecules containing two different atoms like CO, HCl, NO, HBr etc, are called as heteronuclear diatomic molecules.
Write Lewis symbols for the following atoms and ions:
S and S2–; Al and Al3+; H and H–.
Lewis symbol of following atoms and ions ,
S = 2, 8, 6
(Z = 16)