Explain why?
(c) Air pressure in a car tyre increases during driving.
During driving, due to motion of the car the temperature of air inside the tyre increases.
According to Charle's law, P ∝T.
Therefore, air pressure inside the tyre increases.
231 Views

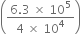

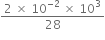 R
R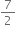 × 8.3
× 8.3 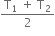 , only when thermal capacities of the two bodies are equal.
, only when thermal capacities of the two bodies are equal.
