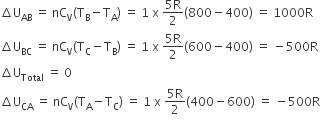Consider a spherical shell of radius R at temperature T. The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume ![]() and pressure.
and pressure.![]() If the shell now undergoes an adiabatic expansion the relation between T and R is
If the shell now undergoes an adiabatic expansion the relation between T and R is
T ∝ e-R
T ∝ e-3R
T ∝ (1/R)
T ∝(1/R3)
C.
T ∝ (1/R)
According to given equation,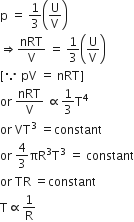
A solid body of constant heat capacity 1 J/°C is being heated by keeping it in contact with reservoirs in two ways:
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies the same amount of heat.
(ii) Sequentially keeping in contact with 8 reservoirs such that each reservoir supplies the same amount of heat. In both the cases body is brought from an initial temperature 100°C to final temperature 200°C. Entropy change of the body in the two cases respectively is:
ln2,4ln2
ln2,ln2
ln2,2ln2
2ln2,8ln2
B.
ln2,ln2
Since entropy is a state function, therefore a change in entropy in both the processes must be same .
The above p-v diagram represents the thermodynamic cycle of an engine, operating with an ideal monoatomic gas. The amount of heat extracted from the source in a single cycle is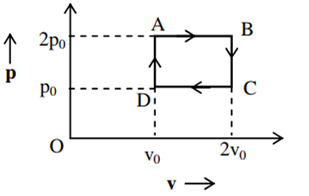
povo
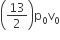
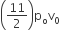
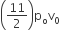
B.
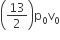
Heat is extracted from the source in path DA and AB is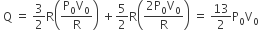
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion the average time of collision between molecules increases as Vq , where V is the volume of the gas. The value of q is: 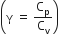
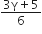
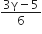
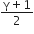
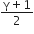
C.
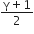
For an adiabatic process TVγ-1 = constant
We know that average time of collision between molecules
Thus, we can write
n =K1V-1 and Vrms = K2T1/2
Where K1 and K2 are constants.
For adiabatic process TVγ-1 = constant. Thus we can write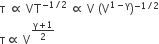
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in the figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement:
The change in internal energy in the process AB is -350 R.
The change in internal energy in the process BC is -500 R.
The change in internal energy in the whole cyclic process is 250 R.
The change in internal energy in the whole cyclic process is 250 R.
D.
The change in internal energy in the whole cyclic process is 250 R.
According to first law of thermodynamics,
(i) change in internal energy from A to B i.e,