
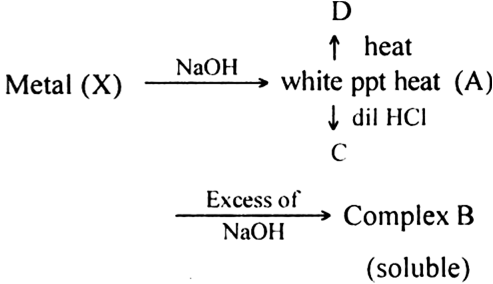
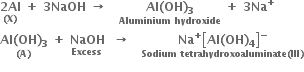
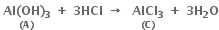
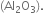

In some of the reactions, thallium resembles aluminium whereas in others it resembles with group 1 metals. Support this statement by giving some evidences.
Thallium resembles aluminium:
(i) Al shows a uniform oxidation state of + 3 and Tl also shows +3 oxidation states in its certain compounds such as TlCl3, Tl2O3 etc.
(ii) Both Al and Tl form octahedral complexes [AlF6]3– and [TlF6]3– respectively.
Thallium resembles group 1 metals:
(i) Thallium (Tl) and group 1 metals show an oxidation state of + 1. Thallium shows an oxidation state of + 1 due to inert pair effect in some of its compounds like Tl2O, TICl6 TIClO4 etc.
(ii) Like group 1 oxides, Tl2O is strongly basic.
