Aluminium trifluoride is insoluble in anhydrous HF but dissolves on the addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through. Give reasons.
(i) Anhydrous HF, being a covalent compound and is strongly H-bonded, therefore it does not give ions. Hence AlF3 does not dissolve in HF. On the other hand, NaF is an ionic compound and gives F– ions and hence AlF3 dissolves in NaF forming soluble complex

(ii) Borax has much higher tendency to form complexes than aluminium because of its smaller size and higher electronegativity. Hence when gaseous BF3 is bubbled through the resulting solution. AlF3 gets precipitated. 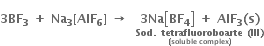
Explain why is there a phenomenal decrease in ionisation enthalpy from carbon to silicion.
When borax is heated strongly, a transparent glass bead which consists of sodium metaborate (NaBO2) and boric anhydride is formed.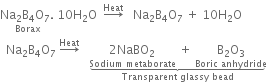
Explain what happens when boric acid is added to water?
Boric acid acts as a weak Lewis acid by accepting a hydroxide ion of water and releasing a proton into the solution. 
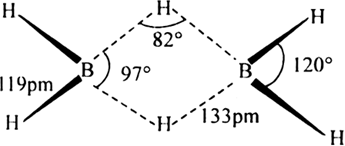
Explain structures of diborane. Write the structures of diborane and explain the nature of bonding in it ?
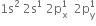 . There are three half filled orbitals in its valence shell. If each boron atom in B2H6 forms three covalent bonds, 14 electrons are required (six B-H bonds and one B– B bond). But there are only 12 electrons (six from two boron atoms and six from hydrogen atoms). So B2H6molecule is short of 2 electrons. Therefore, it cannot have a structure similar to that of C2H6 (ethane).
. There are three half filled orbitals in its valence shell. If each boron atom in B2H6 forms three covalent bonds, 14 electrons are required (six B-H bonds and one B– B bond). But there are only 12 electrons (six from two boron atoms and six from hydrogen atoms). So B2H6molecule is short of 2 electrons. Therefore, it cannot have a structure similar to that of C2H6 (ethane).