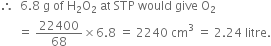Calculate the strength of 10 volume solution of hydrogen peroxide.
Hydrogen peroxide decomposes on heating as,
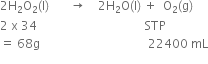
We know,
22400 mL of O2 at STP are produced from H2O2 = 68 g 10 ml of O2 at STP would be produced from H2O2 =
10 ml of O2 at STP would be produced from H2O2 = 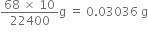
But 10 ml of oxygen at STP are produced from 1 ml of 10 volume of H2O2 solution.
Thus 1 ml of 10 volume at H2O2 solution contains = 0.03036 g H2O2. 1000 ml of 10 volume of H2O2 solution
1000 ml of 10 volume of H2O2 solution
would contain = 0 .03036 x 1000 = 30.36g H2O2 Strength of 10 volume H2O2 = 30.36g L-1
Strength of 10 volume H2O2 = 30.36g L-1
How is strength of hydrogen peroxide expressed? Calculate the strength of 20 volume hydrogen peroxide in grams per litre.
Strength of hydrogen peroxide:
(i) As percentage. It is expressed as W/V percentage of H2O2 in solution. Thus, 30% solution of H2O2 means 30 grams of H2O2 are present in 100 ml of solution.
(ii) In terms of volume. The strength of hydrogen peroxide is expressed in terms of volume i.e. the bottles containing hydrogen peroxide are generally marked as 10 volumes or 20 volumes or 30 volumes etc. A solution of hydrogen peroxide labelled as 20 volume actually means that 1 ml of hydrogen peroxide solution on decomposition by heat produces 20 ml of oxygen at NTP.
Let us now calculate the strength of 20 volume H2O2 in gram/litre.
Hydrogen peroxide decomposes on heating as: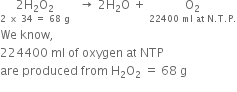

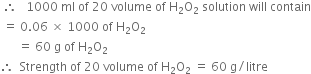
What is the mass of hydrogen peroxide present in 1 litre of 2M solution? Calculate the volume of oxygen at STP liberated upon the complete decomposition of 100 cm3 of the above solution.
(i) To calculate the mass of H2O2 in 1 litre of 2 M solution:
Moleuclar mass of H2O2 = 2 x 1 + 2 x 16 = 34 amu
Now 1 litre of 1 M solution contains
H2O2 = 34 g
(ii) To calculate the volume of O2 liberated at STP from 1000 cm3 of 2M solution:
Decomposition of H2O2 can be represented as:

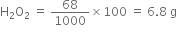
Now 68 g of  at S.T.P. given O2 = 22400 cm3
at S.T.P. given O2 = 22400 cm3