The pair in which phosphorous atoms have a formal oxidation state of +3 is:
Pyrophosphorous and hypophosphoric acids
Orthophosphorous and hypophosphoric acids
Pyrophosphorous and pyrophosphoric acids
Pyrophosphorous and pyrophosphoric acids
D.
Pyrophosphorous and pyrophosphoric acids
Orthophosphorous H3PO4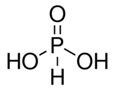
H3PxO3 = 3 +x + 3(-2) = 0 x = +3
pyrophosphorous acids H4P2O6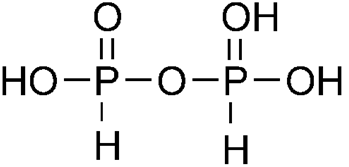
H4P2xO5 = 4 + 2x + 5 (-2) = 0
4 + 2x -10 = 0
x = +3
(a) Account for the following:
(i) Zr and Hf have almost similar atomic radii.
(ii) Transition metals show variable oxidation states.
(iii) Cu+ ion is unstable in aqueous solution.
(b) Complete the following equations:
(i) 2 MnO2 + 4 KOH + O2--->
(ii) 2 Na2CrO4 + 2 H + -->
(i) Due to lanthanide contraction, Zr and Hf have almost similar atomic radii. It can be explained on the basis of shielding effect. The electrons present in inner shells, shield the outer electrons from the nuclear charge, making them experience a low effective nuclear charge. The shielding effect exerted by the electrons decreases in the orders > p > d > f. The f subshell poorly shields the outer electrons from nuclear attraction, which results in the most attractive pull of nucleus on the outer electron. In the case of post lanthanide elements like Hf, 4f subshell is filled and it is not very effective at shielding the outer shell electrons. Therefore, Zr and Hf have almost similar atomic radii.
(ii) The transition metals have their valence electrons in (n-1)d and ns orbitals. Since there is very little energy difference between these orbitals, both energy levels can be used for bond formation. Thus, transition elements exhibit variable oxidation states.
(iii) Cu2+ is more stable than Cu+ in an aqueous medium. Cu2+ has high hydration energy that compensates for the energy required to remove one electron from Cu+to form Cu2+. So in an aqueous medium, Cu+ gives Cu2+ and Cu.
Chemical reaction: 2 Cu+(aq) à Cu2+(aq) + Cu(s)
(b)
(i) 2 MnO2 + 4 KOH + O2 ---> 2 K2MnO4 + 2 H2O
(ii) 2 Na2CrO4 + 2 H+ ----> Na2Cr2O7 + 2 Na+ + H2O
(a) Give reasons for the following:
(i) Mn3+ is a good oxidising agent.
(ii) ![]() Values are not regular for first row transition metals (3d series).
Values are not regular for first row transition metals (3d series).
(iii) Although ‘F’ is more electronegative than ‘O’, the highest Mn fluoride is MnF4, whereas the highest oxide is Mn2O7.
(b) Complete the following equations:
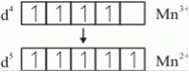
(a) Outer electronic configuration f Mn is 3d5 4s2.
Outer electronic configuration of Mn3+ is 3d4 4s0.
Now Mn3+ is a strong oxidising agent. A good oxidizing agent reduces itself . i.e. gains electrons from other. Its tends to gain one more electron to acquire stable electronic configuration. If it gains one electron, its configuration will be 3d5, which is stable .this is the reason, it acts as a good reducing agent.
(ii) Values are not regular which can be explained by the irregular variation of ionisation enthalpies i.e. iH1 + iH2 and also the sublimation enthalpies which are relatively much less for manganese and vanadium.
(iii) The ability of oxygen to stabilise the higher oxidation state exceeds that of fluorine. Also, the ability of oxygen to form multiple bonds with metals favours Mn2O7. Therefore, the highest Mn fluoride is MnF4 whereas highest oxide is Mn2O7. In Mn2O7, each Mn is tetrahedrally surrounded by O's including a Mn—O—Mn bridge.
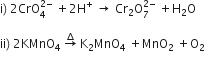
|
|
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
|
-0.91 |
-1.18 |
-0.44 |
-0.28 |
-0.25 |
-0.34 |
From the given data of E0 values answer the following Question:
(i) Which is strongest why is value exceptionally positive?
(ii) Why is value highly negative as compared to other elements?
(iii) Reducing agent Cr2+ or Fe2+? Give reason
(b) Why do actinoids show wide range f oxidation states? Write on the similarity between the chemistry of lanthanoids and actinoids.
The E0 (M2+/M) value of a metal depends on the energy changes involved in the formation of the M2+ ion:
1. Sublimation: The energy required for converting one mole of an atom from the solid state to the gaseous state
M(s) ---> M (g) sH (Sublimation energy)
2. Ionisation: The energy required to take out electrons from one mole of atoms in the gaseous state to form the corresponding cation in the gaseous state
M (g) --->M2+ (g) iH (Ionization energy)
3. Hydration: The energy released when one mole of ions are hydrated
M (g)---> M2+ (aq) hydH (Hydration energy)
Now, copper has high energy of atomization and low hydration energy. Hence, theE0(Cu2+/Cu) is exceptionally positive.
(ii) The  value is highly negative as compared to other elements because of the extra stability of Mn2+ due half -filled d orbitals.
value is highly negative as compared to other elements because of the extra stability of Mn2+ due half -filled d orbitals.
(iii) The following reactions are involved when Cr2+ and Fe+ act as reducing agent:
Cr2+ ---> Cr3+ + e-
Fe2+ ----> Fe3+ + e-
The 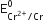 value is -0.41 V and
value is -0.41 V and 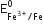 is + 0.77V. This means that Cr2+ can be easily oxidised to Cr3+ but Fe2+ does not get oxidised to Fe3+ easily, therefore, Cr2+ is a stronger reducing agent than Fe3+.
is + 0.77V. This means that Cr2+ can be easily oxidised to Cr3+ but Fe2+ does not get oxidised to Fe3+ easily, therefore, Cr2+ is a stronger reducing agent than Fe3+.
(b) In actinoids, the 5f, 6d, 7s shells are present. These three shells are of comparable energies; therefore electrons can remove from these shells. This gives rise f variable oxidation states in actinoids.
Following are the similarities between actinoids and lanthanoids:
(i) The size of atom: It decreases across the series in both actinoids (due to actinoid contraction) and lanthanoids(due to lanthanoids contraction).
(ii) Oxidation states: Lanthanoids and actinoids generally show +3 oxidation states. However, some element in the actinoids series is capable of exhibiting oxidation states higher than +3.
(a) Why do transition elements show variable oxidation states?
(i) Name the element showing the maximum number of oxidation states among the first series of transition metals from Sc (Z = 21) to Zn (Z = 30).
(ii) Name the element which shows only +3 oxidation state.
(b) What is lanthanoid contraction? Name an important alloy which contains some of the lanthanoid metals.
(a) The ability of the transition metal to exhibit variable valency is generally attributed to the availability of more electrons in the (n-1)d orbitals which are closer to the outermost ns orbital in energy levels.
Thus in the case of iron, we get the divalent Fe(II) state when only the 2 electrons in the 4s orbital are removed. And we get the trivalent Fe(III) state when one more 3d electron is removed, in addition to the two 4s electrons from the neutral Fe atom.
(i) Mn shows a maximum number of oxidation states among the first series of transition metals from Sc to Zn. Mn exhibits all the oxidation states from +2 to +7.
(ii) Scandium shows only +3 oxidation state.
(b) The regular decrease in the size of the atoms and ions with increasing atomic number is known as lanthanide contraction. It arises because as we move along the lanthanide series, the nuclear charge increases by one unit at each successive element, the new electron is added into the same subshell (viz., 4f). As a result, the attraction on the electrons by the nucleus increases and this tends to decrease the size. Further, as the new electron is added into the f-subshell, there is imperfect shielding of one electron by another in this subshell due to the shapes of these f-orbitals. This imperfect shielding is unable to counterbalance the effect of the increased nuclear charge. Hence, the net result is a contraction in the size though the decrease is very small.
