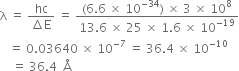A doubly ionised lithium atom is hydrogen-like with atomic number 3:
(i) Find the wavelength of the radiation required to excite the electron in Li++ from the first to the third Bohr orbit. (Ionisation energy of the hydrogen atom equals 13.6 eV.)
(ii) How many spectral lines are observed in the emission spectrum of the above excited system?
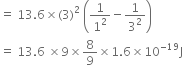
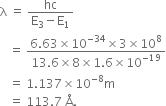
(ii) The following three spectral lines are observed due to the following transitions:
3rd to 1st orbit
3rd to 2nd orbit
2nd to 1st orbit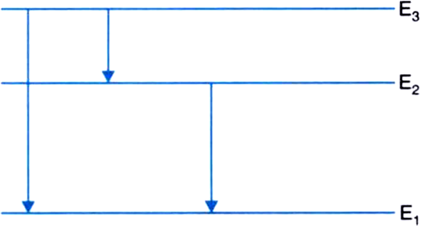
The ground state energy of hydrogen atom is - 13.6 eV.
(i) What is the potential energy of an electron in the 3rd excited state?
(ii) If the electron jumps to the ground state from the 3rd excited state, calculate the wavelength of the photon emitted.
The energy of an electron in nth orbit is given by,
(i) For 3rd excited state, n = 4
Energy at the third excited level is given by,
(ii) Required energy to jump electron to the ground state from the 3rd excited state is,
∴ Wavelength of the photon emitted is,
is the wavelength of the photon emitted.
A single electron, orbits around a stationary nucleus of charge ze, where z is a constant and e is the electronic charge. It requires 47.2 eV to excite the electron from the second Bohr orbit to 3rd Bohr orbit. Find,
(i) The value of z.
(ii) The energy required to excite the electron from the third to the fourth Bohr orbit.
(iii) The wavelength of electromagnetic radiation required to remove the electron from the first Bohr orbit to infinity.
(iv) The kinetic energy, potential energy and angular momentum of the electron in the first Bohr orbit.
(v) The radius of the first Bohr orbit.
(Ionisation energy of hydrogen atom = 13.6 eV. Bohr radius = 5.3 × 10–11 m, velocity of light = 3 × 108 m/s and Planck's constant = 6.6 × 10–34 Js)