
Effect of pressure on adsorption. The extent of adsorption of a gas per unit mass of adsorbent (x / m) increases with increase of pressure at a constant temperature.
(i) For a lower range of pressure (x / m) is directly proportional to the applied pressure. Larger the pressure more is the amount of gas adsorbed, lower the pressure small is the amount of a gas adsorbed. That is![]() (as a constant temperature)
(as a constant temperature)
(ii) For a high pressure range the extent of adsorption of a gas per unit mass of the adsorbent (x / m) is independent of the applied pressure. That is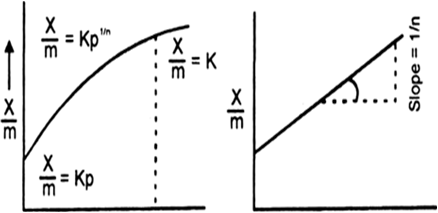
(iii) For a moderate pressure range the value of x/m is proportional to a Fractional power of pressure. That is![]() (where T is constant)
(where T is constant)
where 1 / n is a fraction. Its value may be between 0 and 1. Fig. (a) and (b) show the variations of the extent of adsorption of a gas on an adsorbent as predicted by Frundlich equations (i) and (ii) respectively.
![]() ...(i)
...(i)
and ![]() ...(ii)
...(ii)
Effect of temperature on adsorption: The amount of a gas adsorbed per unit mass of a solid surface (x / m) decreases with increase of temperature in case of physical adsorption. However, in case of chemical adsorption as the temperature increases x / m increases, attains a maximum value then decreases.
Answer:
Enzymes are biological catalysts which increases the rate of cellular reactions.
Mechanism of enzyme catalysis : Enzymes are proteins (globular proteins) and have large molecular masses ranging from 12000 to 40,000. Thus, these are much bigger than the molecules which they catalyse. The substances which are catalysed are known as substrates. The mechanism of the enzyme catalysed reaction is completed in the following steps:
Step-1. Binding enzyme to substrate (reactant) to form a complex.
Step-2. Product formation in the complex:
Step-3. Release of the product from the enzyme complex.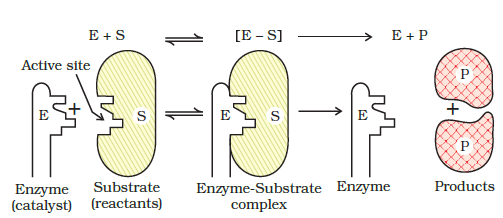
Actually there are a number of cavities present on the surface of the molecules of enzymes. These cavities have specific shapes and contain in their active groups such as – NH2, – COOH, – SH, – OH etc. These function as active sites on the surface of enzyme. The molecules of the reactant (substrate) which have complementary shapes fit into these cavities in the same manner as a key fits into a lock. This results in an activated complex which breaks to give the product and releases the enzyme catalyst.
Answer:
A dispersed phase or dispersion medium in colloidal solution may be a solid, liquid or gas. Based on physical states, 8 types of colloidal systems are possible (gas in gas is not possible as gases are always miscible in all proportions forming homogeneous mixure). All other takes of combinations of gases, liquids and solids may exist as colloidal solutions. Examples are given in the following table:
|
Internal phase of Dispersed phase |
External phase or Dispersion medium |
Colloidal name |
Example |
|
Solid |
Solid |
Solid sols |
Alloys, Ruby glass, Gems or precious stones, marbles, optical and vision glasses. |
|
Liquid |
sols |
Muddy water, gold sol, protein, starch, agar, gelatin in water, paints, pigments in water. |
|
|
Gas |
Aerosols (or solids |
Smoke, particulate clouds. |
|
|
Liquid |
Solids |
Gels |
Cheese, jems, jellies, plants, fruits, vegetables |
|
Liquid |
Emulsions |
Butter, milk, cosmetic products, e.g., shampoo, creams, emulsified oils, polish and medicines. |
|
|
Gas |
Aerosols (or liquids) |
Fog, clouds, mist. |
|
|
Gas |
Solid |
Solid foams |
Pumicestone, styrene foam, foamed rubber, porous pot. thermocole rubber pillows and mattresses. |
|
Liquid |
Foams and froths |
Lather, soap seeds, air bubble. |
|
|
Gas |
Homogeneous system |
Do not exist as colloids. |
Answer:
On the basis of attraction between dispersed phase and dispersion medium colloids may be classified into lyophillic sol and lyophobic sol.
If dispersed phase (solid) tends to attract (i.e., like or love) dispersion medium (liquid), the resultant sol is termed as lyophilic sol. Examples are: Sol of gum, gelatine, starch etc. are lyophilic sols.
On the other hand if dispersed phase tends to repel (i.e., dislike or hatred) dispersion medium, the resultant sol is termed as lyophobic sol. Examples are: Sols of metals, metal hydroxides, metal sulphides etc. are lyophobic sols.
Hydrophobic sols easily coagulated as in this dispersion medium has no apparent affinity or interaction with the dispersed phase.
Answer:
Multimolecular colloids:
(i) Consist of aggregates of atoms or molecules with diameter less than 10–9 m.
(ii) The atom or molecules are held together by weak Vander Waals forces.
Macromolecular colloids:
(i) Are themselves large. Many behave as macromolecular colloids because of their large molecular masses.
(ii) Since the molecules are flexible, they can take on various shapes.
Associated colloids:
(i) Behave as true solution in smaller concentrations and colloids at higher concentrations.
(ii) Sodium stearate behaves as strong electrolyte in dilute solutions and shows the properties of sodium and stearate ions form aggregates and behave as colloids. The aggregates formed are called micelles which revert to individual ions on dilution.
Example of multi-molecular colloid: Gold sol, Sulphur sol.
Example of macro-molecular colloid:Cellulose, Starch.
